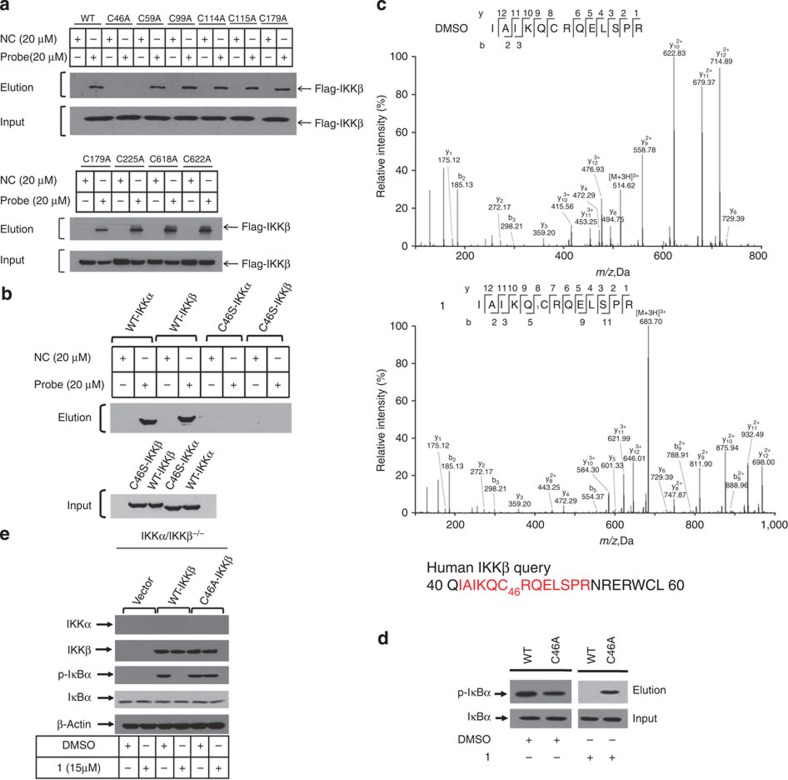
Figure 5. Cysteine 46 of IKKβ is critical for its binding to ainsliadimer A (1).
(a) Recombinant WT-IKKβ and IKKβ-mutant proteins were incubated with Probe or NC at 37 °C for 1.5 h, followed by pull-down with streptavidin-agarose; the precipitates were then resolved by SDS–PAGE and blotted for biotin or flag. (b) Recombinant WT-IKKα, WT-IKKβ, C46S-IKKα or C46S-IKKβ was incubated with Probe or NC at 37 °C for 1.5 h, followed by pull-down with streptavidin-agarose; the precipitates were then resolved by SDS–PAGE and blotted for biotin or Flag. (c) MS/MS analysis of the recombinant IKKβ incubated with or without ainsliadimer A (1) for 3.5 h. (d) Effects of ainsliadimer A (1) on phosphorylation of IκBα in vitro using WT-IKKβ or C46A-IKKβ. Purified Flag-IκBα was used as the substrate and the indicated recombinant proteins were used as kinases. The mixtures were incubated with DMSO or ainsliadimer A (10 μM) for 1 h at 37 °C. Immunoblotting using the p-IκBα antibody reflects the kinase activity and the effects of ainsliadimer A (1). (e) IKKα/IKKβ−/− double-knockout MEF cells were grown to 50% confluence, transfected with 200 ng of the indicated expression plasmids for 8 h, and then treated with ainsliadimer A (1) for 1.5 h. Shown are immunoblots of the total cell lysates using the indicated antibodies.