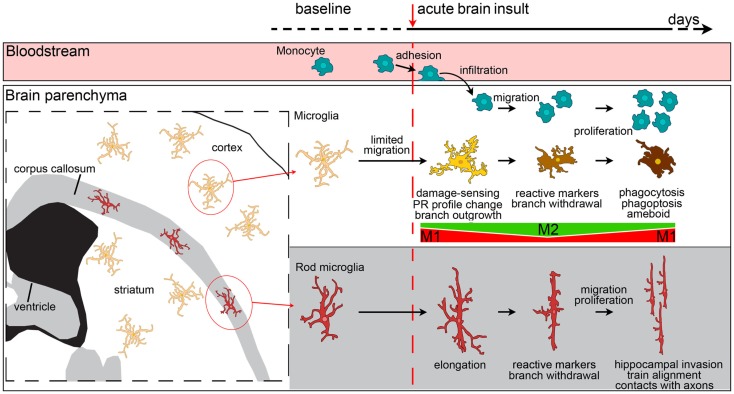
Figure 3.
Evolution of myeloid cell morphology and phenotype in the brain during the acute phases of brain injury. Under physiological conditions, monocytes, microglia and rod microglia populate specific compartments, respectively blood, gray matter and white matter. After an insult to the brain, monocytes, microglia, and rod microglia are activated. Monocytes migrate to lesioned areas, whereas gray matter microglia are less likely to displace. Microglia change morphology in a time-dependent fashion, sprouting new ramifications soon after injury, and then withdrawing branches to develop an ameboid phenotype. Both infiltrated monocytes and microglia change their phenotypical profile, acquire specific functional commitments, and proliferate with time. In the very early phases after injury, microglia have a M1 phenotype, then, with the recruitment of macrophages, both myeloid populations upregulate M2 markers. The peak of M2 marker expression soon vanishes and is followed by upregulation of M1 markers that lasts longer. In vivo, mixed polarization phenotypes are observed and the M1-M2 definition may only serve to set the extremes of a continuum of polarization states. Macrophages from blood-borne monocytes seem to be more able to express M2 markers than microglia. In the white matter, rod microglia activate after injury by enhancing their elongated morphology, expressing reactive markers (CD68, MHCII), and migrating to other areas, such as the hippocampus. Rod microglia also cluster into trains of cells that align to neuronal axons. Neither the exact phenotype profile nor the functions of activated rod microglia are fully understood, though a role of these cells in synaptic stripping due to their contacts with axons has been hypothesized.