Figure 3.
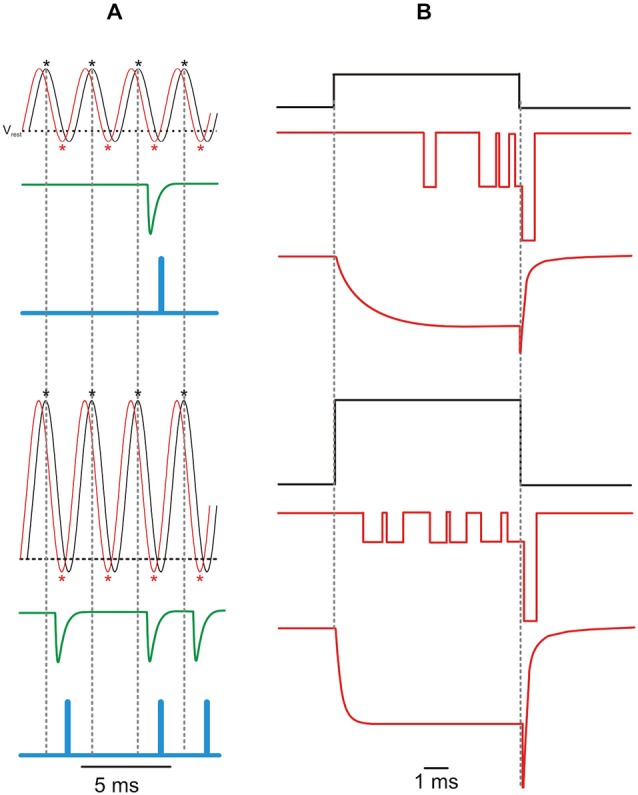
Phase-locking and the Ca2+ current. (A) Idealized traces showing a sinusoidal receptor potential (VMET; black line) and resulting Ca2+ current (ICa; red line) in response to a 400 Hz sound wave with a low (upper panel) and high (lower panel) intensity. The horizontal dashed line indicates the adult mouse IHC resting membrane potential (Vrest = −60 mV; Johnson et al., 2011). Asterisks indicate peak of ICa (red) and of VMET depolarization (black). The resulting increase in intracellular [Ca2+] elicits phase-locked M-EPSCs (green trace; M-EPSC re-drawn from Glowatzki and Fuchs, 2002) which are encoded in action potentials at the primary auditory afferents (blue trace). One EPSC can trigger only one spike (Rutherford et al., 2012), while no vesicles would be released during interspike intervals (i.e., spike discharge would reflects vesicle release probability; Moezzi et al., 2014). As a result of the increased Ca2+ channel Po with IHC depolarization, louder sounds (bottom panel) elicit more action potentials (less failures) with the same timing (phase-locking), as indicated by the vertical dashed lines. Note that, as shown in the bullfrog auditory papilla (Figure 5 in Li et al., 2014), ICa phase-lags VMET, while M-EPSCs only occur in the VMET repolarizing phase. Action potentials further lag VMET due to the time required by electrotonic currents to depolarize the encoder region up to the spiking voltage threshold (~0. 5 ms; Rutherford et al., 2012). (B) Idealized elementary and macroscopic Ca2+ currents elicited by a voltage step from the resting membrane potential of −70 mV to −50 mV (top) or −20 mV (bottom). Activation kinetics of the macroscopic current re-drawn from Johnson and Marcotti (2008), deactivation kinetics re-drawn from Zampini et al. (2014). Increasing IHC depolarization reduces the latency and the time-to-peak of iCa and ICa, while it decreases or increases the amplitude of iCa or ICa, respectively.
