Abstract
Objective
We have retrospectively analyzed the results of the operations made for aortic valve endocarditis in a single center in 26 years.
Methods
From June 1985 to January 2011, 174 patients were operated for aortic valve endocarditis. One hundred and thirty-eight (79.3%) patients were male and the mean age was 39.3±14.4 (9-77) years. Twenty-seven (15.5%) patients had prosthetic valve endocarditis. The mean duration of follow-up was 7.3±4.2 years (0.1-18.2) adding up to a total of 1030.8 patient/years.
Results
Two hundred and eighty-two procedures were performed. The most frequently performed procedure was aortic valve replacement with mechanical prosthesis (81.6%). In-hospital mortality occurred in 27 (15.5%) cases. Postoperatively, 25 (14.4%) patients had low cardiac output and 17 (9.8%) heart block. The actuarial survival rates for 10 and 15 years were 74.6±3.7% and 61.1±10.3%, respectively. In-hospital mortality was found to be associated with female gender, emergency operation, postoperative renal failure and low cardiac output. The long term mortality was significantly associated with mitral valve involvement. Male gender was found to be a significant risk factor for recurrence in the follow-up.
Conclusion
Surgery for aortic valve endocarditis has significant mortality. Emergency operation, female gender, postoperative renal failure and low cardiac output are significant risk factors. Risk for recurrence and need for reoperation is low.
Keywords: Treatment Outcome, Aortic Valve, Endocarditis
Abstract
Objetivo
Analisamos, retrospectivamente, os resultados das operações realizadas para endocardite valvar aórtica em um único centro em 26 anos.
Métodos
De junho de 1985 a janeiro de 2011, 174 pacientes foram operados por endocardite da válvula aórtica. Cento e trinta e oito (79,3%) pacientes eram do sexo masculino e a média de idade foi de 39,3 ± 14,4 (9-77) anos. Vinte e sete (15,5%) pacientes apresentavam endocardite na prótese valvar. O tempo médio de acompanhamento foi de 7,3 ± 4,2 anos (0,1- 18,2) totalizando 1.030,8 paciente/ano .
Resultados
Duzentos e oitenta e dois procedimentos foram realizados. O procedimento mais realizado foi a substituição da valva aórtica por prótese mecânica (81,6 %). A mortalidade intra-hospitalar ocorreu em 27 (15,5%) casos. No pósoperatório, 25 (14,4% ) pacientes apresentaram baixo débito cardíaco e 17 (9,8%) bloqueio cardíaco . As taxas de sobrevida atuarial para 10 e 15 anos foram 74,6±3,7% e 61,1±10,3%, respectivamente. A mortalidade intra-hospitalar foi encontrada esteve associada com o sexo feminino, operação de emergência, insuficiência renal pós-operatória e baixo débito cardíaco. A mortalidade a longo prazo foi significativamente associada com o envolvimento da válvula mitral. O sexo masculino encontrado mostrou-se um fator de risco para a recorrência no seguimento.
Conclusão
A cirurgia para tratamento da endocardite da válvula aórtica apresenta mortalidade. Operação de emergência, o sexo feminino, insuficiência renal pós-operatória e baixo débito cardíaco são fatores de risco significativos. O risco de recorrência e necessidade de reoperação são baixos.
INTRODUCTION
Microbial infection of the endothelial lining of any part of the heart, infective endocarditis (IE), is an uncommon but life threatening condition. Despite advances in diagnosis, antimicrobial therapy, surgical techniques, and management of complications, the incidence has not decreased in the past 30 years and patients with IE still have high morbidity and mortality rates related to this condition [1].
Its management aims to eradicate the infecting organism as soon as possible mainly with antibioticotherapy but clinical complications and treatment failure suggest surgery up to 60% of the cases [2]. IE effecting aortic valve accounts about 40-67% of all cases and about 60-70% of these cases undergo surgery in the acute phase [3]. In spite of the high mortality and morbidity it carries, surgical therapy is still the mainstay in the treatment of aortic valve IE. Numerous studies have assessed different risk factors for mortality and morbidities in the treatment of IE but risk factors for surgical treatment of aortic valve IE patients need to be clarified.
In this study, we have retrospectively assessed the results of the surgical treatment of patients with aortic valve endocarditis over a period of 26 years in an attempt to address these issues.
METHODS
The study was approved by the local hospital ethics committee. The patient data were collected from the hospital records retrospectively. From June 1985 to January 2011, 174 consecutive patients with aortic valve endocarditis underwent surgery at our institution. For the definitions of active, healed, native, and prosthetic and culture negative endocarditis, modified Aranki criteria have been used [4]. The presence of acute or chronic inflammatory changes at microscopy confirmed the diagnosis of endocarditis.
There were 138 (79.3%) male and 36 (20.7%) female patients aged 39.3±14.4 (9-77) years in average. One hundred and forty-seven (84.5%) patients were presented with native valve endocarditis (NVE) and 27 (15.5%) with prosthetic valve endocarditis (PVE). Vegetations on the mitral valve were detected in 38 (21.8%) cases and peri-prosthetic leakage (PPL) was present in 13 patients. Thirty-two (18.4%) patients had a history of previous cardiac surgery and four of them had two cardiac operations previously. Coronary angiography was rarely performed in order to avoid any embolic complications. Of the 27 patients with septic emboli, one of them had both peripheral and central emboli preoperatively. The preoperative characteristics are summarized in Table 1. Culture-negative endocarditis was present when no microorganism could be identified either on serial blood cultures or on cultures from the explanted valvular tissue in patients presenting with the clinical picture of endocarditis. The results of the microbiologic studies can be seen in Table 2.
Table 1.
Preoperative characteristics.
| Preoperative characteristic | n (%) or mean±SD | P1 | P2 | P3 | P4 |
|---|---|---|---|---|---|
| Age | 39.3±14.4 | ns | ns | ns | ns |
| Male / Female | 138 (79.3%) / 36 (20.7%) | 0.022 | 0.034 | ns | ns |
| Fever | 97 (55.7%) | ns | ns | ns | |
| Septic emboli | 27 (15.5%) | ns | ns | ns | ns |
| Central | 14 (8.0%) | ||||
| Peripheral | 14 (8.0%) | ||||
| NYHA Class | |||||
| Class I | 15 (8.6%) | ||||
| Class II | 64 (36.8%) | ||||
| Class III | 74 (42.5%) | ||||
| Class IV | 21 (12.1%) | ||||
| Congestive heart failure | 97 (55.7%) | ns | ns | ns | ns |
| Renal dysfunction | 15 (8.6%) | ns | ns | ns | ns |
| Periprosthetic leakage | 13 (7.5%) | ns | ns | ns | |
| Previous cardiac surgery | 32 (18.4%) | ns | ns | ns | ns |
| Mitral valve involvement | 72 (41.4%) | ns | ns | ns | 0.009 |
| Aortic annulus involvement | 42 (24.1%) | ns | ns | ns | ns |
| Prosthetic valve endocarditis | 27 (15.5%) | ns | ns | ns | ns |
| Operation in the active phase | 84 (48.3%) | ns | ns | ns | ns |
| Culture positive endocarditis | 92 (52.9%) | ns | ns | ns | ns |
| LVD (EF<40%) | 11 (6.3%) | ns | |||
| Emergency operation | 26 (14.9%) | 0.003 | ns | ns | ns |
| ECG | |||||
| Sinus rhythm | 154 (88.5%) | ||||
| AF | 13 (7.5%) | ||||
| AV block | 4 (2.3%) | ||||
| LBBB | 2 (1.1%) | ||||
| RBBB | 1 (0.6%) |
AF= Atrial fibrillation; AV= Atrioventricular; ECG= Electrocardiography; LBBB= Left bundle branch block; LVD= Left ventricular dysfunction; NYHA= New York Heart Association; RBBB= Right bundle branch block; SD= Standard deviation; ns= Not Significant. P1: Statistical significance for in-hospital mortality in logistic regression analysis. P2: Statistical significance for recurrence in logistic regression analysis. P3: Statistical significance for reoperation in logistic regression analysis. P4: Statistical significance for late mortality in logistic regression analysis
Table 2.
Culture results.
| Microorganism | n (%) |
|---|---|
| Culture negative | 82 (47.1%) |
| Streptococcus | 51 (29.3%) |
| Staphylococcus | 23 (13.2%) |
| Brucella | 11 (6.3%) |
| MRSA | 4 (2.3%) |
| Acinetobacter | 1 (0.6%) |
| E. Coli | 1 (0.6%) |
| Enterobacter | 1 (0.6%) |
MRSA: Methicillin resistant Staphylococcus aureus; E. coli: Escherichia coli
The diagnosis of IE was performed according to the Duke criteria [5]. All patients were examined by transthoracic or transesophageal echocardiography. Valvular destruction was evident in 120 (69.0%) cases and aortic annular involvement was present in 42 (24.1%) cases. Gross vegetations on aortic valves were detected in 25 (14.4%) patients and vegetations on mitral valve in 38 (21.8%) cases preoperatively. Operations were performed during the active phase of infection in 84 (48.3%) patients. The indications for emergency surgery were high as well as mobile vegetations on the aortic valve, acute leaflet rupture and cardiac decompensation, periannular extensive abscess with intracardiac fistula and prosthetic valve dysfunction.
Operative technique
All patients underwent moderate (28ºC) hypothermic cardiopulmonary bypass by means of bicaval cannulation with cannulation of either the ascending aorta (169 patients) or the femoral artery (5 patients). The left ventricle was vented through the right superior pulmonary vein. Isothermic blood cardioplegic solution was administered via antegrade and retrograde route during aortic cross-clamping.
For eradication of the aortic valve endocarditis, radical debridement of all the necrotic and infected tissues was performed. In cases with annular involvement, aortic annulus was skeletonized. All infected and necrotic tissue around the annulus and when present, within the abscess and fistula between the ventriculoarterial junction and the sinotubular junction were resected. All vegetations were removed. When the aortic valve was not extensively damaged, vegetectomy and reconstruction either primary or by using pericardial patch was preferred as described before [6]. Before cardiopulmonary bypass, a patch was harvested from the pericardium, stabilized with 0.62% glutaraldehyde solution for 5 minutes, and rinsed thoroughly with 0.9% saline solution. When necessary, the pericardial strip trimmed to an appropriate length and was sutured continuously with 5-0 polypropylene according to the area to be patched. The completely resected annular area was covered with the glutaraldehyde-treated autologous pericardial patch sutured to firm, fibrous tissue for a secure anastomosis or valve implantation [6,7].
Mitral valve involvement was present in 72 (41.4%) cases. Two hundred and eighty-two procedures were performed on 174 patients. The list of procedures can be seen in Table 3. In the cases with aortic PPL, all prostheses were replaced except for four patients that had a primary repair of the periprosthetic leak. Primary repair of the mitral PPL was preferred in two cases.
Table 3.
Procedures.
| Procedures | n (%) * | P1 | P2 | P3 | P4 |
|---|---|---|---|---|---|
| Aortic valve replacement | 142 (81.6%) | ||||
| Redo AVR | 13 (7.5%) | ||||
| AVR with bioprosthesis | 2 (1.1%) | ||||
| Aortic reconstruction | 3 (1.7%) | ||||
| Primary repair of periprosthetic leak | 4 (2.3%) | ||||
| Aortic root replacement | 22 (12.6%) | ||||
| Bentall de Bono procedure | 10 (5.7%) | ||||
| Xenograft implantation | 6 (3.4%) | ||||
| Homograft implantation | 5 (2.9%) | ||||
| Cabrol procedure | 1 (0.6%) | ||||
| Aortic root enlargement | 1 (0.6%) | ||||
| Fistula repair | 6 (3.4%) | ||||
| Patch repair of a sinus Valsalva aneurysm repair | 4 (2.3%) | ||||
| Subaortic discrete membrane resection | 4 (2.3%) | ||||
| Aortic vegetectomy | 3 (1.7%) | ||||
| Drainage of subaortic abscess and patch repair | 2 (1.1%) | ||||
| Septal vegetectomy | 1 (0.6%) | ||||
| Patch repair of an ascending aortic pseudoaneurysm | 1 (0.6%) | ||||
| Graft interposition in the ascending aorta | 1 (0.6%) | ||||
| Patch repair of a ventricular septal defect | 6 (3.4%) | ||||
| Mitral valve procedures | 72 (41.4%) | ||||
| Mitral valve replacement | 55 (31.6%) | ||||
| Redo mitral valve replacement | 4 (2.3%) | ||||
| Mitral reconstruction | 14 (8.0%) | ||||
| Primary repair of periprosthetic leak | 2 (1.1%) | ||||
| Vegetectomy of mitral leaflets | 1 (0.6%) | ||||
| Tricuspid De Vega annuloplasty | 3 (1.7%) | ||||
| Coronary artery bypass grafting | 2 (1.1%) | ||||
| Primary repair of atrial septal defect | 2 (1.1%) | ||||
| Closure of patent ductus arteriosus | 1 (0.6%) | ||||
| Femoral embolectomy | 1 (0.6%) | ||||
| Pericardiectomy | 1 (0.6%) | ||||
| Concomitant procedure | 81 (46.6%) | 0.971 | 0.205 | 0.237 |
Percentages are the ratio to the number of patients. AVR: Aortic valve replacement. P1: Statistical significance for in-hospital mortality in logistic regression analysis. P2: Statistical significance for recurrence in logistic regression analysis. P3: Statistical significance for reoperation in logistic regression analysis. P4: Statistical significance for late mortality in logistic regression analysis
Follow-up
All patients received at least four weeks of antibiotherapy postoperatively. Broad range antibiotics (vancomycin and aminoglycosides) were preferred in culture-negative cases. Antibiotics were arranged according to the antibiograms results in culture-positive patients. After the patients were discharged from the hospital, they were involved in a follow-up program in the outpatient clinic. Follow-up was complete in 95.2% of the patients. Seven patients were lost to follow-up. The mean duration of follow-up was 7.3±4.2 years (0.1-18.2) adding up to a total of 1030.8 patient/years.
Statistical analysis
The statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) 16.0 statistical software package. All continuous variables were expressed as mean ± standard deviation with the ranges and discrete variables as frequencies and percentages. Comparisons of the discrete variables were made by chi-square test or Fisher's Exact test where appropriate. The survival, freedom from recurrence and reoperation analyses was made with Kaplan Meier analysis. Logistic regression analyses were performed for the factors affecting early and late mortality, recurrence and reoperation.
The independent variables for in hospital mortality, recurrence and reoperation were concomitant non-aortic procedure, previous cardiac surgery, septic emboli, preoperative fever, preoperative renal dysfunction, congestive heart failure, PPL, culture-negative endocarditis, operation during active phase, PVE, aortic annular involvement, mitral valve involvement, valvular destruction, emergency operation, postoperative heart block, postoperative renal dysfunction, postoperative fever and low cardiac output (LCO).
The independent variables for late mortality were previous cardiac surgery, septic emboli, preoperative renal dysfunction, congestive heart failure, culture-negative endocarditis, and left ventricular dysfunction, operation during active phase of infection, aortic annular involvement, mitral valve involvement, emergency operation, postoperative heart block, postoperative fever, PVE, recurrence and reoperation. Age and gender adjustments were made. The survival comparisons were made with log-rank test. P values less than 0.05 were accepted as statistically significant differences.
RESULTS
Mortality
Twenty-seven (15.5%) patients had in-hospital mortality. Fourteen were female and 11 had PVE. The reasons for mortality are outlined in Table 4. Emergency operation (OR=4.40; 95% CI: 1.68-11.51; P=0.003), postoperative LCO (OR=1.19; 95% CI: 1.78-76.92; P=0.011), postoperative renal failure (OR=7.52; 95% CI: 1.22-45.45; P=0.030) and female gender (OR=5.62; 95% CI: 1.28-25.00; P=0.022) were associated with in hospital mortality in regression analysis.
Table 4.
Operative variables and postoperative morbidity.
| Perioperative characteristic | n (%) | P1 | P2 | P3 | P4 |
|---|---|---|---|---|---|
| Postoperative fever | 45 (25.9%) | ns | ns | ns | ns |
| Postoperative atrioventricular block | 17 (9.8%) | ns | ns | ns | ns |
| Low cardiac output | 25 (14.4%) | 0.011 | ns | ns | |
| Renal failure | 34 (19.5%) | 0.030 | ns | ns | |
| Need for dialysis | 12 (6.9%) | ||||
| Valvular destruction | 120 (69.0%) | ns | ns | ns | |
| Recurrence during follow-up | 10 (5.7%) | ns | |||
| Reoperation during follow-up | 8 (4.6%) | ns |
ns: Not Significant. P1: Statistical significance for in-hospital mortality in logistic regression analysis. P2: Statistical significance for recurrence in logistic regression analysis. P3: Statistical significance for reoperation in logistic regression analysis. P4: Statistical significance for late mortality in logistic regression analysis
Morbidity
Postoperative fever was found in 45 (25.9%) patients. Twenty-five (14.4%) patients had LCO and 18 cases in this group died in the postoperative follow-up. Complete heart block was present in 17 (9.8%) patients, but only four (2.3%) patients required permanent pacemaker implantation. Four of these patients had complete heart block preoperatively.
Three of these patients had PVE and 6 had aortic annular involvement. Five patients with postoperative heart block died in the early postoperative period. Renal dysfunction was present in 34 (19.5%) patients and 12 (6.9%) required dialysis. Pulmonary morbidity was present in 24 (13.8%) patients. Cerebrovascular events occurred in 8 (4.6%) patients. Seven of these cases underwent surgery in the active phase of infection. Septic central emboli were present in two cases preoperatively. Seventy-three (42%) patients had >2 morbidity postoperatively. Postoperative morbidity has been outlined in Table 5.
Table 5.
Patients with mortality.
| Etiology for mortality | n (%) |
|---|---|
| Hospital mortality | 27 |
| Low cardiac output | 15 (55.6%) |
| Low cardiac output + sepsis | 7 (25.9%) |
| Sepsis | 3 (11.1%) |
| Pulmonary complication | 1 (3.7%) |
| Sudden death | 1 (3.7%) |
| Late mortality | 14 |
| Cardiac | 8 (57.1%) |
| Extracardiac | 6 (42.9%) |
Follow-up
Follow-up was complete in 140 (95.2%) cases. Seven patients were lost to follow-up. Fourteen (8%) patients had mortality after discharge. Three of them were female. The data of the patients with early and late mortality have been summarized in Table 5.
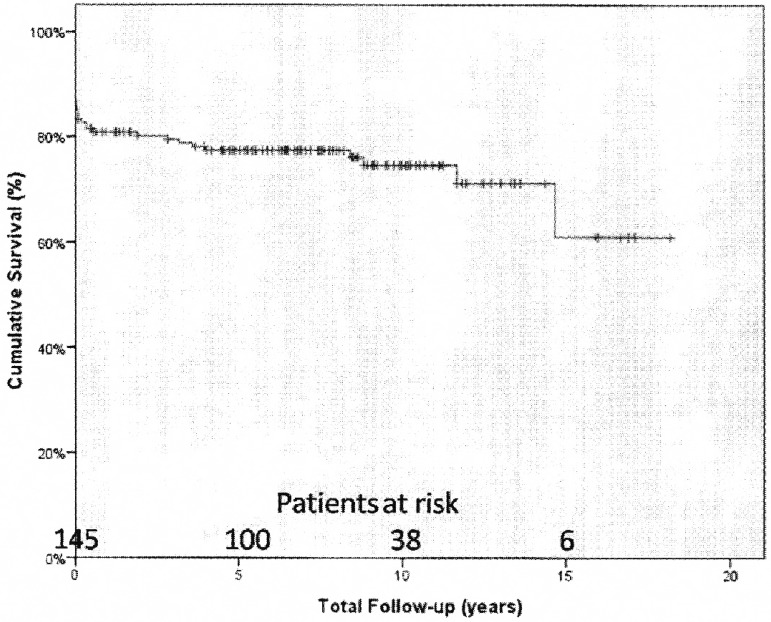
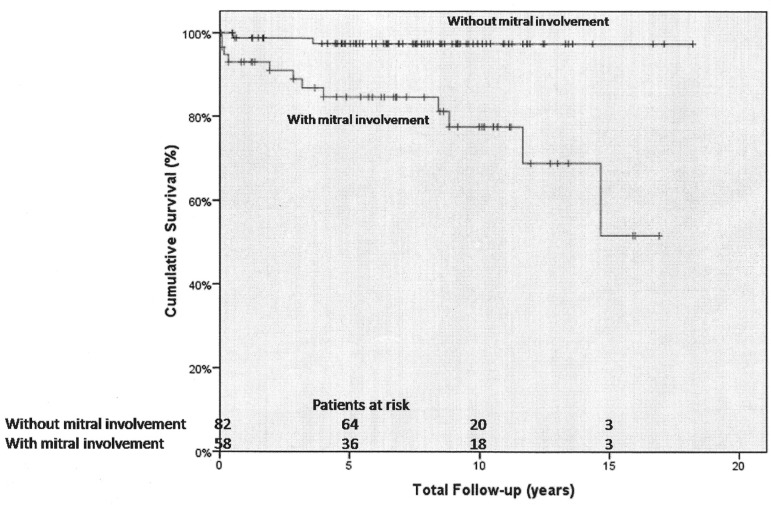
The actuarial survival rates for 1, 5, 10 and 15 years were 80.8%±3%, 77.4%±3.3%, 74.6±3.7% and 61.1±10.3%, respectively (Figure 1). The logistic regression analysis showed that mitral valve involvement (OR=45.45; 95% CI: 2.56-1000.00; P=0.009) was associated with long term mortality and the significance persisted after adjustment for sex and age. The difference in the actuarial survival rates in patients with and without mitral valve involvement were also statistically significant (P=0.0001). The 1, 5, 10 and 15 years survival rates for patients with mitral valve involvement were 96.6%±2.4%, 84.6%±5%, 77.5%±6.7% and 51.7%±16.7%, respectively. The 1 and 5 years survival rates for patients without mitral valve involvement were 98.8%±1.2% and 97.4%±1.8% and remained stable for the following term (Figure 2).
Fig. 1.
Actuarial survival curve
Fig. 2.
Actuarial survival curve for patients with and without mitral valve involvement
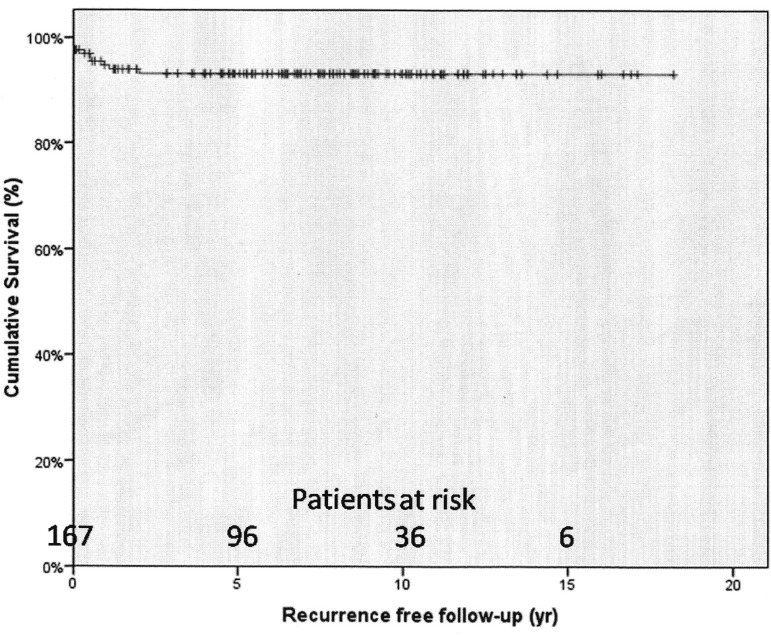
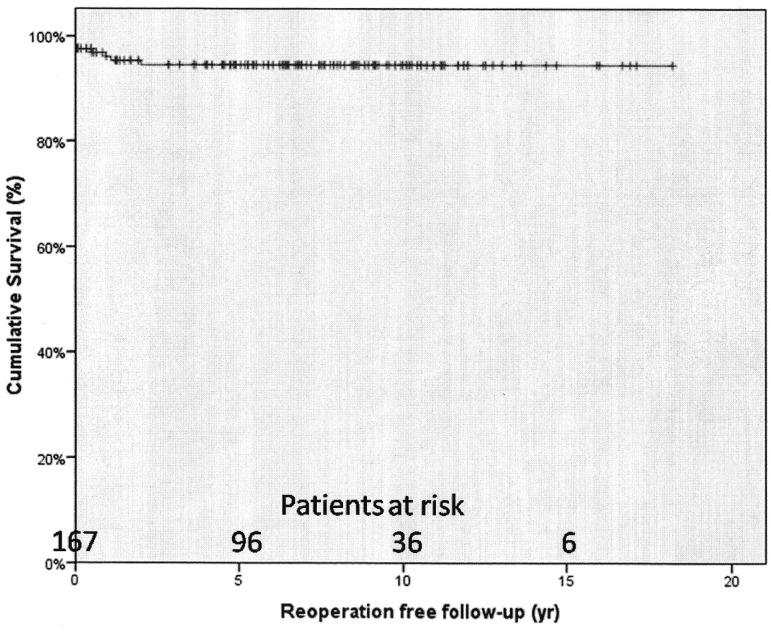
Recurrence of infection occurred in 10 (5.7%) cases and reoperations were performed in 8 (4.6%). The rates of freedom from recurrence from infection at 1 and 5 years were 94.7%±1.8% and 93.1%±2.1% (Figure 3). The rates of freedom from reoperation at 1 and 5 years were 96.1%±1.6% and 94.5%±1.9%, respectively (Figure 4). Male gender was significantly associated with recurrence (OR=9.35; 95% CI: 1.41-16.85; P=0.034), however, none of the factors were found to be associated with reoperation.
Fig. 3.
Survival free from recurrence
Fig. 4.
Survival free from reoperation
DISCUSSION
Aortic valve endocarditis is mainly managed surgically since 1965 [8]. The disease is highly fatal for the potential consequences of sepsis and cardiac dysfunction. This study is a report of a single institution experience for aortic valve endocarditis over 26 years. The majority of the cases were operated for aortic valve replacement with mechanical prosthesis.
Despite the advances in the operative and postoperative techniques, surgery is still associated with a significant mortality rate. The latest study about the results of surgery for IE in America reports 17% overall mortality [9]. There is a wide range of variation in mortality rates ranging from 6% to 33% [2,10-14]. We have previously reported 12% mortality for active IE [15]. The slight increase (15.5%) was mainly because of the patient profile. In our previous report, congestive heart failure was present in less than 40% of the cases, however, in this group, more than 55% of the cases had congestive heart failure. Another striking difference is the lack of PVE cases in the previous report. In fact, regression analysis did not give PVE as a risk factor for in-hospital mortality. Delay et al. [16] did not observe significant differences as well. A recent study from Sweden reported 30-days mortality for surgically treated native and PVE as 5.2% and 14.7% respectively, but did not mention PVE as a risk factor [13].
However, it may affect the overall mortality in many different ways. Especially the preoperative status is an important factor. Another important determinant was emergency operation. The significant association of emergency operation and in-hospital mortality was not unexpected. Kirali et al. [15] reported significant association of urgent operations in NVE before. The indications for emergency operation in IE were documented with high level of evidence [17]. The poor outcomes were also reported by Revilla et al. [18]. They found that the poor outcomes were associated with persistent infection and renal failure but not with heart failure. More recently, Koeda et al. [12] reported even mild renal dysfunction at the time of admission as an important predictor of early mortality. However, we did not detect any association of renal failure and mortality. Female gender was significantly associated with in-hospital mortality in our analysis like others [19,20].
LCO and postoperative renal dysfunction were found to be associated with in-hospital mortality which suggests that cardiorenal interaction may play an important role in the deterioration of IE. Their associations with postoperative mortality were similar to a recent reports [9,10,21]. Operation in the active phase was not a significant factor for any dependent variable in our analyses (Table 1). Although the relation is not so obvious, it may still be a risk factor for in-hospital mortality. The high rate of active phase operations (almost 50%) in this cohort could be understood this way.
The primary aim of surgery is the eradication of infective tissue and the reconstruction of cardiac morphology. Mode of surgery (replacement/repair) or the type of prosthesis (mechanical/ biologic) was reported to have no influence on mortality [19,22]. Although biological solution is recommended especially for aortic root involvement [23], biological prostheses, especially allografts were reported to have a significant reoperation rate which increases with time [24]. We used mechanical prosthesis in the majority of the patients. The low recurrence and reoperation rates in this study confirm our preference for the valve substitute.
The long-term survival given in this report is compatible with recent reports [9,11]. One of the most interesting results of our analysis was the significant lower survival in patients with mitral valve involvement. The regression analysis revealed that presence of mitral valve involvement was significantly associated with long-term mortality (Table 1) and this association was confirmed by the Kaplan Meier analysis results (Figure 2). Multivalve involvement association with higher mortality was reported before [10], but the lower survival was not mentioned. The most possible explanation may be the extent of the destruction by the infectious process. In the analysis of patients with double valve endocarditis, Gillinov et al. [25] report that increasing age was significantly associated with lower long term survival. Age was not a significant predictor in our analysis.
One of the most important aspects of surgery for IE is the recurrence. The recurrence was 5.7% in our patient group (Table 4). The rate of recurrence is similar to others [26] and the recurrence-free survival is very satisfactory (Figure 3). The only significant association with recurrence was male gender (Table 1). The reoperation-free survival rates were also satisfactory. These satisfactory results were due to the high rate of mechanical valve utilization according to our point of view. This fact was also discussed by others [19,24]. Fedoruk and colleagues reported that type of prosthesis did not affect survival and recurrence rates were primarily associated with human immunodeficiency virus infection and intravenous drug usage [27]. None of the patients in our cohort had such history. Most important feature in preventing recurrence is complete debridement [28]. The low rates of recurrence and reoperation confirm the completeness of the debridement.
The most important drawback of our study is the high rate of culture negative cases. Although no associations with mortality and morbidity were revealed (Table 1), this high negative culture results necessitates further attention. The most frequently growing bacteria was streptococcus (Table 2) in this group, however, we could not reach to further conclusions due to the high rate of negative cultures. In the literature, 20% to 60% incidence of negative culture results have been reported [14,29]. Even United States reported 69.4% positive culture rates using Nationwide Inpatent Sample. High rate of unknown cultures (30.6%) was explained by coding error [30]. Several factors may cause the lack of isolation of microorganisms from the blood like healed endocarditis, broad range antibiotic therapy, low-virulence germs and poor blood sampling [8]. Also, we did not make serological testing for some rare bacteria which could further lower the culture negative rates. Another important limitation is the retrospective nature of the study. We tried to neutralize this fact by detailed multivariable analyses.
CONCLUSION
In conclusion, surgery for aortic valve endocarditis was associated with significant mortality. Emergency operation, female gender, postoperative renal failure and LCO are significant risk factors for in-hospital mortality. Operation in the active phase of infection was a risk factor for in-hospital mortality as it constitutes a significant risk factor for postoperative LCO. Male gender was a predictor for recurrence but no risk factors were found to be significant for reoperation. In the surviving patients, risk for recurrence and need for reoperation was low. Long term survival was lower in patients who had mitral valve involvement.
| Abbreviations, acronyms & symbols | |
|---|---|
| CI | Confidence interval |
| IE | Infective endocarditis |
| LCO | Low cardiac output |
| NVE | Native valve endocarditis |
| OR | Odds ratio |
| PPL | Periprosthetic leakage |
| PVE | Prosthetic valve endocarditis |
| SPSS | Statistical Package for the Social Sciences |
| Authors' roles & responsibilities | |
|---|---|
| TA | Writer |
| EYT | Co-writer |
| ST | Collecting and Analyzing Data |
| AAD | Collecting and Analyzing Data |
| EBP | Analyzing Data |
| AT | Final control of the study |
Footnotes
No financial support.
This study carried out at Kartal Kosuyolu Heart and Research Hospital, Department of Cardiovascular Surgery, Istanbul, Turkey.
REFERENCES
- 1.Bashore TM, Cabell C, Fowler V., Jr Update on infective endocarditis. Curr Probl Cardiol. 2006;31(4):274–352. doi: 10.1016/j.cpcardiol.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Nakatani S, Mitsutake K, Ohara T, Kokubo Y, Yamamoto H, Hanai S, CADRE Investigators Recent picture of infective endocarditis in Japan: lessons from Cardiac Disease Registration (CADRE-IE) Circ J. 2013;77(6):1558–1564. doi: 10.1253/circj.cj-12-1101. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen DT, Delahaye F, Obadia JF, Duval X, Selton-Suty C, Carteaux JP, et al. AEPEI study group Aortic valve replacement for active infective endocarditis: 5-year survival comparison of bioprostheses, homografts and mechanical prostheses. Eur J Cardiothorac Surg. 2010;37(5):1025–1032. doi: 10.1016/j.ejcts.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 4.Aranki SF, Santini F, Adams DH, Rizzo RJ, Couper GS, Kinchla NM, et al. Aortic valve endocarditis. Determinants of early survival and late morbidity. Pt 2Circulation. 1994;90(5):II175–II182. [PubMed] [Google Scholar]
- 5.Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200–209. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 6.Bozbuga N, Erentug V, Erdogan HB, Kirali K, Ardal H, Tas S, et al. Surgical treatment of aortic abscess and fistula. Tex Heart Inst J. 2004;31(4):382–386. [PMC free article] [PubMed] [Google Scholar]
- 7.Kirali K, Omeroglu SN, Mansuroglu D, Ipek G, Yakut C. Aortic root abscess with fistula formation into right ventricular myocardium. Türk Kardiyol Dern Arş. 2000;28:647–649. [Google Scholar]
- 8.Wallace AG, Young WG, Jr, Osterhout S. Treatment of acute bacterial endocarditis by valve excision and replacement. Circulation. 1965;31:450–453. doi: 10.1161/01.cir.31.3.450. [DOI] [PubMed] [Google Scholar]
- 9.Machado MN, Nakazone MA, Murad-Júnior JA, Maia LN. Surgical treatment for infective endocarditis and hospital mortality in a Brazilian single-center. Rev Bras Cir Cardiovasc. 2013;28(1):29–35. doi: 10.5935/1678-9741.20130006. [DOI] [PubMed] [Google Scholar]
- 10.Gaca JG, Sheng S, Daneshmand MA, O'Brien S, Rankin JS, Brennan JM, et al. Outcomes for endocarditis surgery in North America: a simplified risk scoring system. J Thorac Cardiovasc Surg. 2011;141(1):98–106.:98-106.e1-2. doi: 10.1016/j.jtcvs.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Prendergast BD, Tornos P. Surgery for infective endocarditis: who and when? Circulation. 2010;121(9):1141–1152. doi: 10.1161/CIRCULATIONAHA.108.773598. [DOI] [PubMed] [Google Scholar]
- 12.Koeda C, Tashiro A, Itoh T, Okabayashi H, Nakamura M. Mild renal dysfunction on admission is an important prognostic predictor in patients with infective endocarditis: a retrospective single-center study. Intern Med. 2013;52(10):1013–1018. doi: 10.2169/internalmedicine.52.9305. [DOI] [PubMed] [Google Scholar]
- 13.Ternhag A, Cederström A, Törner A, Westling K. A nationwide cohort study of mortality risk and long-term prognosis in infective endocarditis in Sweden. PLoS One. 2013;8(7):e67519. doi: 10.1371/journal.pone.0067519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elbey MA, Akdag S, Kalkan ME, Kaya MG, Sayin MR, Karapιnar H, et al. A multicenter study on experience of 13 tertiary hospitals in Turkey in patients with infective endocarditis. Anadolu Kardiyol Derg. 2013;13(6):523–527. doi: 10.5152/akd.2013.172. [DOI] [PubMed] [Google Scholar]
- 15.Kirali K, Guler M, Yakut N. Combined medical and surgical treatment for active native valve infective endocarditis: ten-year experience. Turk Kardiyol Dern Arş. 2001;29:543–548. [Google Scholar]
- 16.Delay D, Pellerin M, Carrier M, Marchand R, Auger P, Perrault LP, et al. Immediate and long-term results of valve replacement for native and prosthetic valve endocarditis. Ann Thorac Surg. 2000;70(4):1219–1223. doi: 10.1016/s0003-4975(00)01887-7. [DOI] [PubMed] [Google Scholar]
- 17.Delahaye F, Célard M, Roth O, de Gevigney G. Indications and optimal timing for surgery in infective endocarditis. Heart. 2004;90(6):618–620. doi: 10.1136/hrt.2003.029967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Revilla A, López J, Vilacosta I, Villacorta E, Rollán MJ, Echevarría JR, et al. Clinical and prognostic profile of patients with infective endocarditis who need urgent surgery. Eur Heart J. 2007;28(1):65–71. doi: 10.1093/eurheartj/ehl315. [DOI] [PubMed] [Google Scholar]
- 19.Avierinos JF, Thuny F, Chalvignac V, Giorgi R, Tafanelli L, Casalta JP, et al. Surgical treatment of active aortic endocarditis: homografts are not the cornerstone of outcome. Ann Thorac Surg. 2007;84(6):1935–1942. doi: 10.1016/j.athoracsur.2007.06.050. [DOI] [PubMed] [Google Scholar]
- 20.Wallace SM, Walton BI, Kharbanda RK, Hardy R, Wilson AP, Swanton RH. Mortality from infective endocarditis: clinical predictors of outcome. Heart. 2002;88(1):53–60. doi: 10.1136/heart.88.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa MA, Wollmann DR, Jr, Campos AC, Cunha CL, Carvalho RG, Andrade DF, et al. Risk index for death by infective endocarditis: a multivariate logistic model. Rev Bras Cir Cardiovasc. 2007;22(2):192–200. doi: 10.1590/s0102-76382007000200007. [DOI] [PubMed] [Google Scholar]
- 22.Edwards MB, Ratnatunga CP, Dore CJ, Taylor KM. Thirty-day mortality and long-term survival following surgery for prosthetic endocarditis: a study from the UK heart valve registry. Eur J Cardiothorac Surg. 1998;14(2):156–164. doi: 10.1016/s1010-7940(98)00148-1. [DOI] [PubMed] [Google Scholar]
- 23.Dossche K, Brutel de la Rivière A, Morshuis W, Schepens M, Ernst J. Aortic root replacement with human tissue valves in aortic valve endocarditis. Eur J Cardiothorac Surg. 1997;12(1):47–55. doi: 10.1016/s1010-7940(97)00145-0. [DOI] [PubMed] [Google Scholar]
- 24.Klieverik LM, Yacoub MH, Edwards S, Bekkers JA, Roos-Hesselink JW, Kappetein AP, et al. Surgical treatment of active native aortic valve endocarditis with allografts and mechanical prostheses. Ann Thorac Surg. 2009;88(6):1814–1821. doi: 10.1016/j.athoracsur.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Gillinov AM, Diaz R, Blackstone EH, Pettersson GB, Sabik JF, Lytle BW, et al. Double valve endocarditis. Ann Thorac Surg. 2001;71(6):1874–1879. doi: 10.1016/s0003-4975(01)02603-0. [DOI] [PubMed] [Google Scholar]
- 26.Heiro M, Helenius H, Mäkilä S, Hohenthal U, Savunen T, Engblom E, et al. Infective endocarditis in a Finnish teaching hospital: a study on 326 episodes treated during 1980-2004. Heart. 2006;92(10):1457–1462. doi: 10.1136/hrt.2005.084715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fedoruk LM, Jamieson WR, Ling H, Macnab JS, Germann E, Karim SS, et al. Predictors of recurrence and reoperation for prosthetic valve endocarditis after valve replacement surgery for native valve endocarditis. J Thorac Cardiovasc Surg. 2009;137(2):326–333. doi: 10.1016/j.jtcvs.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 28.Renzulli A, Carozza A, Romano G, De Feo M, Della Corte A, Gregorio R, et al. Recurrent infective endocarditis: a multivariate analysis of 21 years of experience. Ann Thorac Surg. 2001;72(1):39–43. doi: 10.1016/s0003-4975(01)02703-5. [DOI] [PubMed] [Google Scholar]
- 29.Renzulli A, Carozza A, Marra C, Romano GP, Ismeno G, De Feo M, et al. Are blood and valve cultures predictive for long-term outcome following surgery for infective endocarditis? Eur J Cardiothorac Surg. 2000;17(3):228–233. doi: 10.1016/s1010-7940(00)00342-0. [DOI] [PubMed] [Google Scholar]
- 30.Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelstein DU. Infective endocarditis in th U.S., 1998-2009: a nationwide study. PLoS One. 2013;8(3):e60033. doi: 10.1371/journal.pone.0060033. [DOI] [PMC free article] [PubMed] [Google Scholar]