Figure 1.
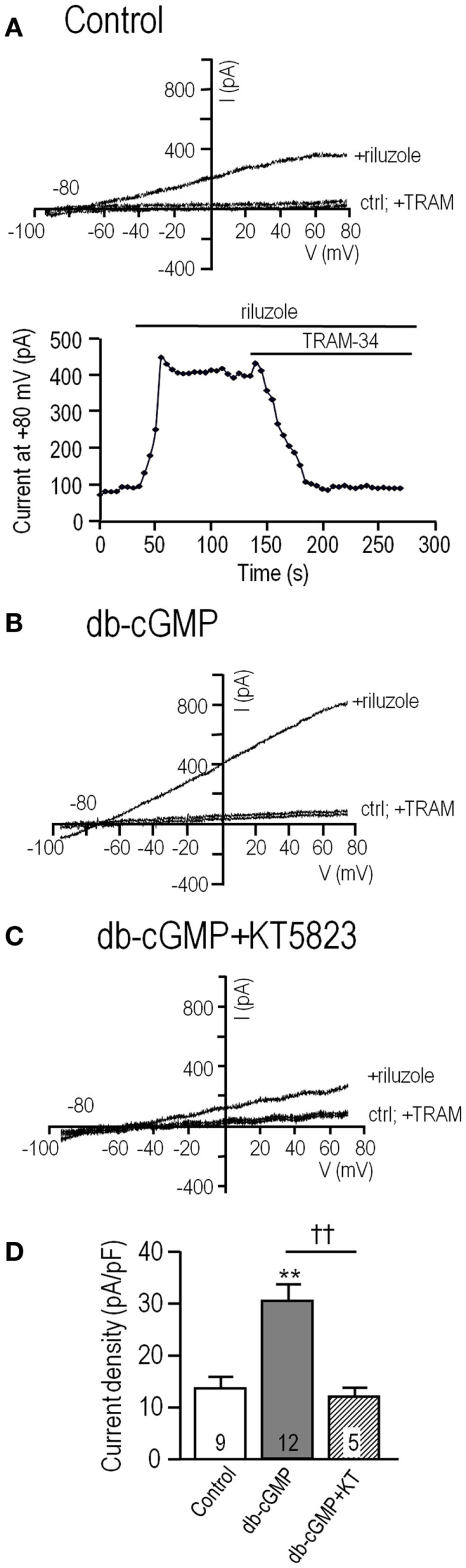
The endogenous KCa3.1 current in MLS-9 microglial cells is increased by cGMP, which requires cGMP-protein kinase. For all traces, the voltage protocol was a holding potential of –70 mV, and repeated ramps from –100 to +80 mV. Recordings were conducted at room temperature in the perforated-patch configuration, and riluzole was used simply to activate the KCa3.1 current at the normal low intracellular Ca2+ concentration. The bath always contained 100 nM apamin, a KCa2.1–2.3 channel blocker. (A) Upper: Representative current traces from a control cell (trace marked “ctrl”), followed by bath addition of 300 μM riluzole, and then 1 μM of the selective KCa3.1 blocker, TRAM-34. Lower: The time course of current activation and block by 1 μM TRAM-34. (B,C) Representative current traces from cells before and after activating the current with riluzole; with or without 1 μM TRAM-34. Cells were pre-treated with the membrane-permeant cGMP analog, db-cGMP (100 μM), for 20 min at room temperature, without (B) or with (C) 1 μM KT5823, a selective inhibitor of cGMP-protein kinase (PKG). (D) Summarized data from a population study using the treatments in panels A–C. For each cell, the KCa3.1 current amplitude was measured at +80 mV, as the component of the riluzole-activated current that was blocked by TRAM-34 (1 μM). The current was always normalized to the cell capacitance (in pF) to account for any differences in cell size and expressed as current density. The TRAM-34-sensitive KCa3.1 current is expressed as mean ± SEM for the number of cells indicated on each bar, and data were compared using one-way ANOVA, with Tukey’s post hoc test. **p < 0.01, indicates a difference from both controls and KT5823-treated cells. ††p < 0.01, for the comparison indicated. There was no difference between the control and KT5823-treated cells.
