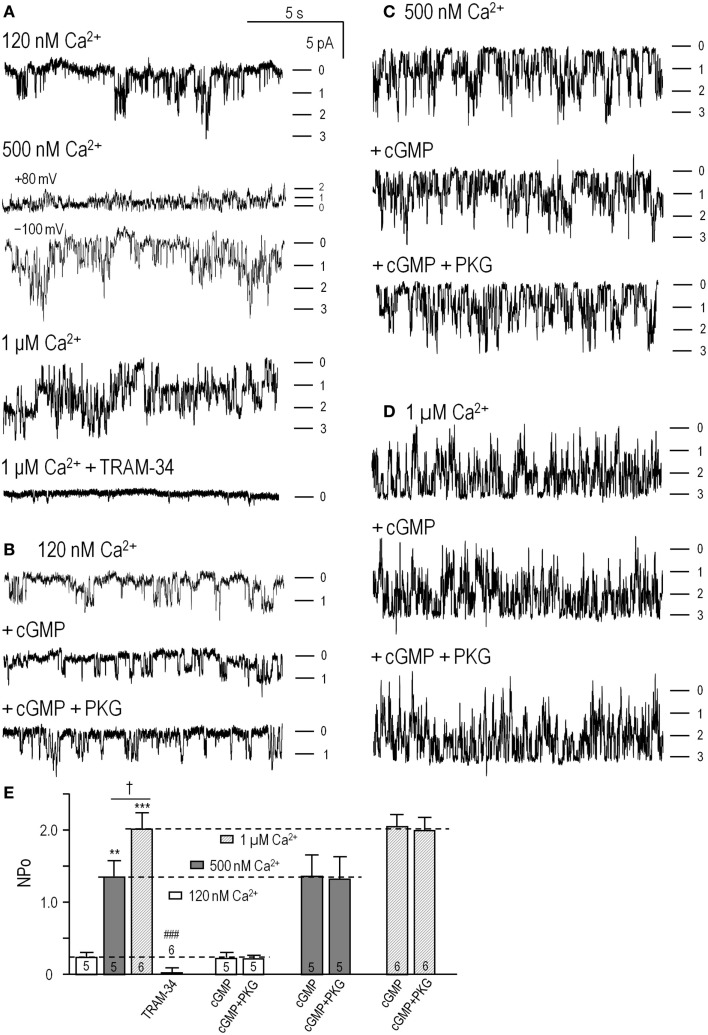
Figure 2.
cGMP-protein kinase (PKG) did not directly affect KCa3.1 channel activity. Inside-out patches were excised from HEK293 cells that had been transfected with wild-type human KCNN4 (KCa3.1). The bath and pipette solutions both contained 140 mM potassium, and unless otherwise indicated, inward single-channel currents were recorded at a membrane potential of −100 mV. (A) KCa3.1 channel activity was recorded with intracellular solutions containing ATP and 120 nM, 500 nM, or 1 μM free Ca2+, sequentially perfused into the bath. At the end of the recording, the KCa3.1 selective blocker, 1 μM TRAM-34, was perfused in. Patches usually contained multiple channels, and the dashes indicate the closed level and opening of 1, 2, or 3 channels. (B–D) At each Ca2+ concentration (120 nM, 500 nM, 1 μM), the bath was sequentially perfused with cGMP (100 μM), and cGMP + PKG holoenzyme (1 U/μL). (E) Summarized data show NPo in control bath solution and 4–6 min after adding cGMP or cGMP + PKG. Data are expressed as mean ± SEM for the number of patches indicated on the bars. The dashed lines indicate the NPo value in control bath solution at each Ca2+ concentration. A two-way ANOVA with Tukey’s post hoc test shows that activity increased with intracellular Ca2+ (**p < 0.01 for 500 nM Ca2+, and ***p < 0.001 for 1 μM Ca2+) and was significantly reduced by 1 μM TRAM-34 (only data for 1 μM Ca2+ shown; ###p < 0.001). There were no differences with cGMP or cGMP/PKG treatments.