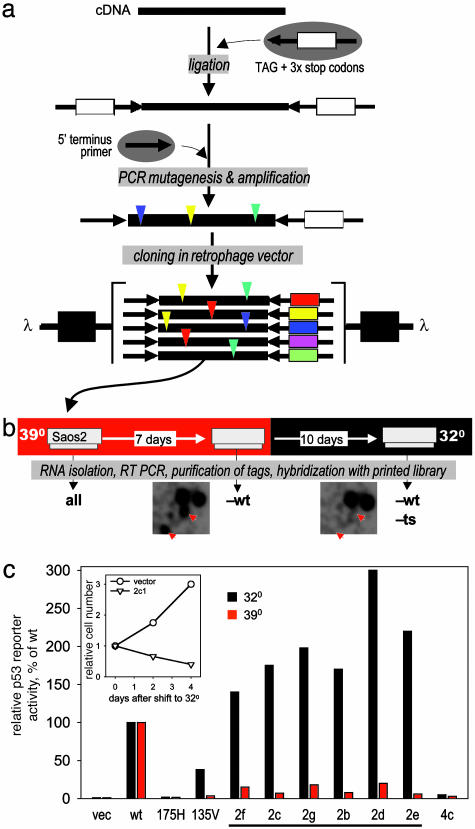
Fig. 4.
Isolation of temperature-sensitive p53 mutants. (a) Construction of library of random p53 mutants. The human p53 cDNA was blunt ended and ligated to the tag adaptor. PCR mutagenesis was performed in the presence of manganese and unequal dNTP pools (11, 12). A 5′ p53-specific primer and a primer specific for the 3′ terminus of the tag adaptor were used to control for cDNA orientation. The resulting tagged mutants were cloned into the retrophage vector, which was used to transduce Saos-2 at 39°C. (b) Selection scheme. Saos-2 were infected with the library at 39°C and passaged for 7 days before being shifted to 32°C for an additional 10 days. The tags were isolated by means of RT-PCR and hybridized. Similar fragments of autoradiograms resulting from hybridization with 32°C and 39°C probes are shown; positions of plaques corresponding to putative temperature-sensitive mutants are shown by red arrows. (c) Confirmation of biological activity for individual clones was assessed by transactivation of a p53 luciferase reporter and toxicity at both 32°C and 39°C as compared with wild type, p53R175H, and mouse p53A135V. Bars represent relative activity of p53-responsive luciferase reporter at two different temperatures calculated as a percentage of that of wild-type p53 cloned in the same vector and determined after transient transfection of Saos-2 cells. Underlined clones displayed predicted ts mutants. Clone 4c was picked as an example of a putative nonfunctional mutant (tag was not lost at any temperature). (Inset) Results of cell growth estimation of Saos-2 cells transduced with one of the ts mutants (2c) after temperature shift from 39° to 32°C. Mutations relative to wild-type human p53 are indicated in Table 1.