Abstract
Background
Whether intraoperative use of hydroxyethyl starch impairs kidney function remains unknown. The authors thus tested the primary hypothesis that Hextend promotes renal injury in surgical patients. Secondarily, the authors evaluated the dose–outcome relationship, in-hospital and 90-day mortality, and whether the relationship between colloid use and acute kidney injury (AKI) depends on baseline risk for AKI.
Methods
The authors evaluated the data of 44,176 adults without preexisting kidney failure who had inpatient noncardiac surgery from 2005 to 2012. Patients given a combination of colloid and crystalloid were propensity matched on morphometric, and baseline characteristics to patients given only crystalloid. The primary analysis was a proportional odds logistic regression with AKI as an ordinal outcome based on the Acute Kidney Injury Network classification.
Results
The authors matched 14,680 patients receiving colloids with 14,680 patients receiving noncolloids for a total of 29,360 patients. After controlling for potential confounding variables, the odds of developing a more serious level of AKI with Hextend was 21% (6 to 38%) greater than with crystalloid only (P = 0.001). AKI risk increased as a function of colloid volume (P < 0.001). In contrast, the relationship between colloid use and AKI did not differ on baseline AKI risk (P = 0.84). There was no association between colloid use and risk of in-hospital (P = 0.81) or 90-day (P = 0.02) mortality.
Conclusion
Dose-dependent renal toxicity associated with Hextend in patients having noncardiac surgery is consistent with randomized trials in critical care patients.
NEW-ONSET acute kidney injury (AKI) occurs in approximately 1 to 7% of patients having noncardiac surgery depending on the classification of AKI.1,2 It is associated with increased cost, prolonged hospitalization, and worsened morbidity and mortality.3–7 Postoperative patients requiring new renal replacement therapy have an especially high risk of dying.8,9
Perioperative fluid management can be a major determinant of postoperative kidney function.10 Hypovolemia is associated with increases in morbidity and mortality,11 whereas excessive fluid administration can provoke hemodynamic deterioration, pulmonary complications, or tissue edema.12–14 Maintaining physiologic perioperative intravascular volume is thus desirable.11–15 Much evidence suggests that guiding fluid therapy to optimal stroke volume, for example with esophageal Doppler ultrasonography, improves outcome.5,16,17 Nearly all randomized evaluations of goal-directed fluid management used colloids for fluid optimization, most often a hydroxyethyl starch (HES) solution.
Low-molecular-weight HES, though, increased the risk of AKI and mortality in recent large randomized trials,18,19 conclusions that are supported by systematic reviews.20–22 However, these studies largely focused on critically ill patients (i.e., sepsis and major trauma) who were given large volumes of HES. But unlike critical care patients who are often given high-dose HES over prolonged periods, surgical patients are typically given smaller amounts over just a few hours. It remains unknown whether the adverse effects of HES observed in critically ill patients apply to routine surgical patients. We therefore evaluated the association between intraoperative HES administration and postoperative kidney function after noncardiac surgery.
Specifically, we tested the primary hypothesis that intra-operative HES (Hextend; Hospira Inc., Lake Forest, IL) administration increases the risk of new-onset postoperative kidney injury as defined by Acute Kidney Injury Network criteria.23 Secondarily, we tested the hypotheses that: (1) cumulative intraoperative HES volume is independently associated with risk of new-onset postoperative kidney injury; (2) the relationship between HES use and AKI depends on baseline AKI risk; (3) in-hospital mortality is greater in patients given intraoperative HES; and (4) 90-day mortality is greater in patients given intraoperative HES.
Materials and Methods
With approval from our Cleveland Clinic Institutional Review Board (Cleveland, Ohio), we obtained data on 120,406 unique adults who had noncardiac, nonurological inpatient surgery at the Cleveland Clinic between January 2005 and September 2012 from our Perioperative Health Documentation System. The Cleveland Clinic Perioperative Health Documentation System contains all patients who have noncardiac surgery since May 2005 at Cleveland Clinic’s main campus. It integrates preoperative variables (demographics, conditions, etc.), intraoperative variables (via the Anesthesia Record Keeping system), and postoperative outcomes (by linking to the larger Cleveland Clinic billing data systems).
Subject Selection
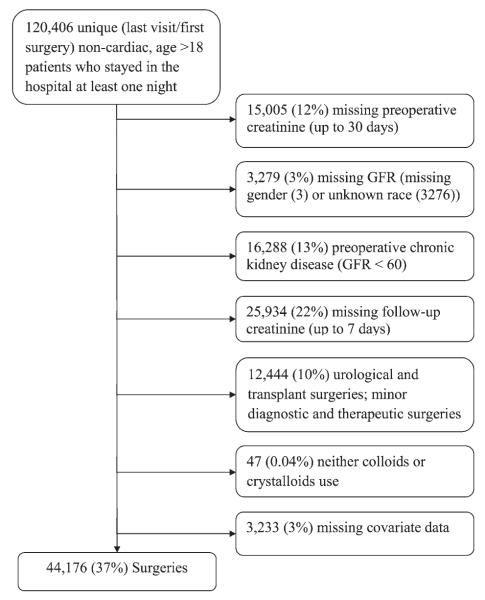
We excluded patients for whom preoperative and postoperative serum creatinine concentrations were unavailable; patients with preoperative chronic kidney disease, defined by an estimated glomerular filtration rate of less than 60 ml min−1 1.73 m−2 as defined by the Modification of Diet in Renal Disease equation24; patients having transplant surgery; and patients not having major diagnostic or therapeutic surgery (as defined by the U.S. Agency for Healthcare Research and Quality’s procedure classes).* Patients with missing covariate values (fig. 1) were also excluded.
Fig. 1.
Flow chart of patient selection. GFR = glomerular filtration rate.
By far, the most common colloid used intraoperatively at the Cleveland Clinic is Hextend, which is a high-molecular-weight (670 kDa) colloid solution with a high molar substitution (0.75) and a concentration of 6%. It has an osmolarity of 307 mOsM, an ionic composition similar to that of Lactated Ringer’s solution, and a maximal daily dose of 20 ml/kg. Only patients given this colloid were considered in our analysis.
As per Walsh et al.,2 we extended the normal 48-h creatinine window used by the Acute Kidney Injury Network classification to 7 days to better characterize the postoperative period. Urine output was not considered. The Acute Kidney Injury Network criterion defines three stages of AKI based on maximum elevations in serum creatinine, resulting in an ordered outcome.
Statistical Analysis
To account for potential confounding due to systematic differences between study groups, we matched each patient who was given both crystalloids and colloids (“colloid” group) to a control patient who was given only crystalloids (“crystalloid” group) on baseline morphometric, and presurgical variables listed in table 1 under Patients’ baseline characteristics. Surgical categories were defined using the U.S. Agency for Healthcare Research and Quality’s single-level Clinical Classifications Software for International Classification of Diseases, 9th Revision, Clinical Modification procedure codes.* Any anesthesiologist or surgical category represented by fewer than 100 patients were combined into respective all-purpose “other” categories for the purposes of modeling.
Table 1.
Patients’ Baseline and Intraoperative Characteristics before and after the Propensity Score Matching
| Before Matching |
After Matching |
|||||
|---|---|---|---|---|---|---|
| Colloids | Noncolloids | Colloids | Noncolloids | |||
|
|
|
|||||
| Variables | (N = 22,289) | (N =21,887) | ASD, %* | (N = 14,680) | (N = 14,680) | ASD, %* |
| Patients’ baseline characteristics | ||||||
| Sex, male, % | 10,061 (45) | 8,757 (40) | 10 | 6,132 (42) | 6,178 (42) | 1 |
| Age, yr | 58 ± 15 | 55 ± 16 | 19 | 56 ± 15 | 56 ± 16 | 0 |
| Body mass index, kg/m2 | 29 [24, 34] | 28 [24, 33] | 10 | 28 [24, 34] | 28 [24, 34] | 0 |
| Race, % | ||||||
| Black | 2,618 (12) | 2,803 (13) | 4 | 1,820 (12) | 1,805 (12) | 1 |
| Other | 356 (2) | 401 (2) | 268 (2) | 249 (2) | ||
| White | 19,315 (87) | 18,683 (85) | 12,592 (86) | 12,626 (86) | ||
| Diabetes, % | 3,695 (17) | 3,455 (16) | 2 | 2,404 (16) | 2,439 (17) | 1 |
| Diabetes with insulin therapy, % | 887 (4) | 855 (4) | 0 | 580 (4) | 595 (4) | 1 |
| COPD, % | 3,068 (14) | 2,980 (14) | 0 | 1,997 (14) | 2,026 (14) | 1 |
| Ascites, % | 313 (1) | 206 (1) | 4 | 186 (1) | 181 (1) | 0 |
| Active congestive heart failure, % | 822 (4) | 840 (4) | 1 | 542 (4) | 548 (4) | 0 |
| Hypertension, % | 11,289 (51) | 9,714 (44) | 13 | 6,888 (47) | 6,899 (47) | 0 |
| Vascular occlusive disease, % | 4,173 (19) | 4,018 (18) | 1 | 2,598 (18) | 2,629 (18) | 1 |
| Asthma, % | 1,818 (8) | 1,913 (9) | 2 | 1,234 (8) | 1,267 (9) | 1 |
| Autoimmune disease, % | 125 (1) | 165 (1) | 2 | 95 (1) | 86 (1) | 1 |
| ARB, % | 797 (4) | 531 (2) | 7 | 443 (3) | 422 (3) | 1 |
| Diuretic medications, % | 2,458 (11) | 1,727 (8) | 11 | 1,323 (9) | 1,332 (9) | 0 |
| Nephrotoxic medications, % | 777 (3) | 424 (2) | 10 | 349 (2) | 346 (2) | 0 |
| ACE inhibitor, % | 2,261 (10) | 1,600 (7) | 10 | 1,227 (8) | 1,218 (8) | 0 |
| Preoperative serum creatinine, mg/dl | 0.82 ± 0.18 | 0.81 ± 0.18 | 9 | 0.81 ± 0.18 | 0.81 ± 0.18 | 0 |
| Preoperative hemoglobin, g/dl | 13.30 ± 1.80 | 13.08 ± 1.88 | 12 | 13.19 ± 1.83 | 13.18 ± 1.85 | 0 |
| Emergency surgery, % | 710 (3) | 1,078 (5) | 9 | 584 (4) | 577 (4) | 0 |
| Intraperitoneal surgery, % | 9,240 (41) | 10,043 (46) | 9 | 6,765 (46) | 6,716 (46) | 1 |
| Year of surgery | ||||||
| 2005 | 1,145 (5) | 1,003 (5) | 34 | 680 (5) | 679 (5) | 1 |
| 2006 | 2,679 (12) | 2,220 (10) | 1,625 (11) | 1,608 (11) | ||
| 2007 | 2,914 (13) | 2,056 (9) | 1,618 (11) | 1,649 (11) | ||
| 2008 | 3,210 (14) | 2,274 (10) | 1,816 (12) | 1,791 (12) | ||
| 2009 | 3,735 (17) | 2,947 (13) | 2,283 (16) | 2,268 (15) | ||
| 2010 | 3,843 (17) | 3,500 (16) | 2,574 (18) | 2,547 (17) | ||
| 2011 | 2,665 (12) | 4,485 (20) | 2,277 (16) | 2,306 (16) | ||
| 2012 | 2,098 (9) | 3,402 (16) | 1,807 (12) | 1,832 (12) | ||
| ASA status, % | ||||||
| I | 358 (2) | 550 (3) | 12 | 281 (2) | 283 (2) | 1 |
| II | 8,170 (37) | 8,731 (40) | 5,640 (38) | 5,609 (38) | ||
| III | 12,411 (56) | 11,061 (51) | 7,797 (53) | 7,841 (53) | ||
| IV | 1,349 (6) | 1,541 (7) | 961 (7) | 945 (6) | ||
| Kheterpal AKI risk class, % | ||||||
| I | 13,324 (60) | 14,225 (65) | 11 | 9,142 (62) | 9,144 (62) | 0 |
| II | 5,283 (24) | 4,531 (21) | 3,284 (22) | 3,270 (22) | ||
| III | 2,631 (12) | 2,266 (10) | 1,622 (11) | 1,626 (11) | ||
| IV/V | 1,051 (5) | 865 (4) | 632 (4) | 640 (4) | ||
| Anesthesiologist† | 5 | 0 | ||||
| Category of surgery‡ | 70 | 4 | ||||
| Arthroplasty knee | 2,337 (10) | 1,288 (6) | 1,201 (8) | 1,201 (8) | ||
| Colorectal resection | 1,746 (8) | 1,239 (6) | 1,151 (8) | 1,100 (7) | ||
| Other OR lower GI therapeutic procedures | 847 (4) | 2,258 (10) | 838 (6) | 896 (6) | ||
| Spinal fusion | 2,021 (9) | 750 (3) | 773 (5) | 746 (5) | ||
| Hip replacement; total and partial | 2,335 (10) | 568 (3) | 528 (4) | 568 (4) | ||
| Hysterectomy; abdominal and vaginal | 1,082 (5) | 1,600 (7) | 1,004 (7) | 956 (7) | ||
| Other OR upper GI therapeutic procedures | 1,296 (6) | 1,183 (5) | 956 (7) | 960 (7) | ||
| Incision and excision of CNS | 877 (4) | 1,392 (6) | 853 (6) | 847 (6) | ||
| Other OR gastrointestinal therapeutic procedures |
1,322 (6) | 636 (3) | 631 (4) | 623 (4) | ||
| Other OR therapeutic nervous system procedures |
625 (3) | 937 (4) | 603 (4) | 611 (4) | ||
| Laminectomy; excision intervertebral disc | 924 (4) | 716 (3) | 662 (5) | 634 (4) | ||
| Aortic resection; replacement or anastomosis |
690 (3) | 342 (2) | 317 (2) | 325 (2) | ||
| Other hernia repair | 373 (2) | 572 (3) | 344 (2) | 348 (2) | ||
| Other OR procedures on vessels other than head and neck |
433 (2) | 513 (2) | 373 (3) | 376 (3) | ||
| Small bowel resection | 506 (2) | 374 (2) | 342 (2) | 335 (2) | ||
| Intraoperative characteristics§ | ||||||
| Hypotension, % | 7,950 (36) | 4,153 (19) | 5,388 (37) | 2,944 (20) | ||
| Blood loss, ml | 250 [100, 500] | 75 [30, 150] | 200 [100, 440] | 100 [50, 200] | ||
| Duration of surgery, min | 264 [203, 354] | 198 [149, 258] | 265 [206, 353] | 205 [157, 264] | ||
| Urine output, ml | 375 [175, 700] | 200 [0, 485] | 380 [175, 720] | 220 [0, 500] | ||
| Transfusion of erythrocytes, % of patients∥ | 3,342 (15) | 909 (4) | 2,068 (14) | 682 (5) | ||
| Amount of erythrocytes, ml# | 699 [371, 1,061] | 649 [356, 774] | 699 [372, 1,061] | 657 [359, 783] | ||
| Vasopressor use, %∥ | 16,037 (72) | 9,601 (44) | 10,270 (70) | 6,573 (45) | ||
Results presented as means ± SDs for normally distributed variables, median [first quartile, third quartile] for skewed data, or N (%) for proportions. The two groups were imbalanced on 15 of 26 baseline potential confounders (ASD >0.05) before matching; however, all patient baseline variables were well balanced after matching.
ASD (colloids minus noncolloids): absolute value of the difference in means or proportions divided by pooled SD converted into percentages; ASD above 5% in absolute value indicates imbalance.
We did not list the anesthesiologist ID due to privacy reason.
Listed are top 15 (approximately 78% of total) categories of surgical procedures.
We did not use intraoperative characteristics for matching. Matching was based on demographic and baseline characteristics.
Intraoperative vasopressor use and intraoperative blood product transfusion were not used as a potential confounders in the analysis; they may be associated with the AKI outcome but were not considered to be confounders because neither is likely to have influenced the decision to administer colloids.
Amount of intraoperative blood product transfusion reported for the subsets of patients who received erythrocytes transfusion.
ACE = angiotensin-converting enzyme; AKI = acute kidney injury; ARB = angiotensin receptor blockers; ASA status = American Society of Anesthesiologists physical status classification; ASD = absolute standardized differences; CNS = central nervous system; COPD = chronic obstructive pulmonary disease; GI = gastrointestinal; OR = operating room.
We excluded intraoperative potential confounding variables from matching because we directly adjusted for those variables when comparing matched groups on outcome. This approach allowed us to conduct the sensitivity analyses discussed below. Specifically, we excluded intraoperative estimated blood loss, intraoperative hypotension, intraoperative urine output, and estimated duration of surgery (table 1).
Matching was implemented on the basis of the propensity score (i.e., the estimated probability of colloid administration, as a function of the baseline potential confounding variables) using a greedy distance-based matching algorithm. Propensity scores were estimated via multivariable logistic regression. Matching was restricted to pairs within 0.2 SDs of the propensity score logits (i.e., within 0.2 × 0.9469 = 0.1894) of one another.25 Except where specified, all reported analyses used this subset of matched surgeries.
When adjusting for confounding, care must be taken to not “over-adjust” for variables that may be mechanisms by which colloid administration causes increased risk of AKI. We therefore a priori distinguished potential confounders from potential mediators. The former being variables that potentially influence both fluid use and AKI, and the latter referring to variables that are mechanisms by which colloid administration might increase the risk of AKI—that is, variables that are potentially caused by colloid administration and that also affect AKI.
We assumed that intraoperative estimated blood loss, intraoperative hypotension, urine output, and duration of surgery were potential confounders and adjusted for each factor in all analyses (fig. 2). In contrast, intraoperative vasopressor use (use of either of dobutamine, dopamine, epinephrine, norepinephrine, phenylephrine, or vasopressin) and intraoperative blood product transfusion (erythrocyte transfusion) may be associated with the AKI outcome1 but were not considered to be confounders. This was based on our assumption that the administration of HES is mainly triggered by blood loss, and that administration of transfusions and vasopressors happens thereafter and thus might not influence the decision to administer colloids. Thus, vasopressor use and blood transfusions might be mediators. We therefore did not adjust for these two variables in the main analyses. However, we also performed several sensitivity analyses using vasopressors and transfusions as confounders as it is almost impossible to distinguish whether they might be mediators or confounders (see second paragraph under primary outcome).
Fig. 2.
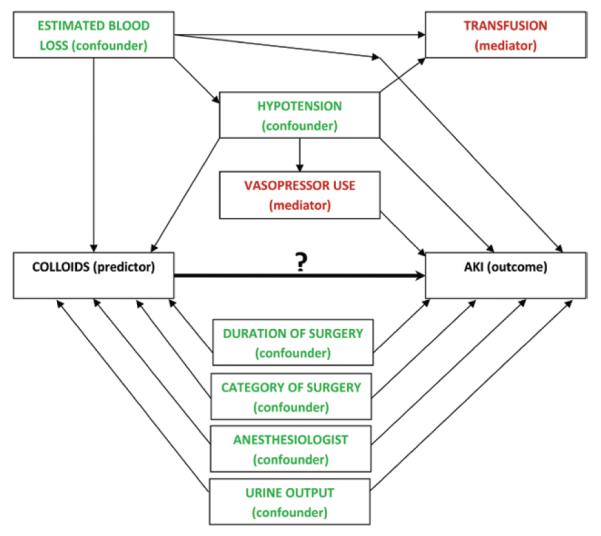
Intraoperative variables and their assumed causal relationships with the exposure and outcome. Potential confounding variables (green) are those that might influence both hydroxyethyl starch administration and acute kidney injury (AKI): estimated blood loss, intraoperative hypotension, urine output, duration and category of surgery, and the anesthesiologist. Potential mediator variables, such as intraoperative vasopressor use and blood product transfusion (red), might be external variables that are mechanisms by which hydroxyethyl starch administration might cause increased risk of AKI but do not influence the decision to administer hydroxyethyl starch.
Balance between the two study groups on patients’ baseline characteristics was assessed, both before and after matching, using univariable summary statistics (mean and SD for normally distributed variables, median [first quartile, third quartile] for skewed data, or proportions) and absolute standardized difference scores (ASDs). ASDs are defined as the absolute value of the difference between means, mean rankings, or proportions divided by a combined estimate of SD converted into percentages; thus, the ASD roughly represents the percent of SDs by which the two study groups differ. We considered an ASD greater than 5% after matching as indicative of potential residual confounding and subsequently adjusted for such factors directly in all analyses comparing the groups on outcome (in addition to the intraoperative potential confounding variables).
Model-based Wald chi-square tests were used to test all hypotheses involving proportional odds model coefficients. The overall type I error rate for our study was controlled at 5% by evaluating the primary hypothesis at the 2.5% significance level and constraining the overall type I error rate for the secondary hypotheses to 2.5%. The Bonferroni correction for multiple comparisons was used to constrain the overall type I error rate for the secondary hypotheses.
SAS statistical software version 9.3 (SAS Institute, Cary, NC) for 64-bit Microsoft Windows and R statistical software version 2.15.2 for 64-bit Unix operating system (The R Foundation for Statistical Computing, Vienna, Austria) were used for all statistical analyses.
Primary Outcome
Matched patients given both crystalloids and colloids were compared with matched patients given only crystalloids in a proportional odds logistic regression model with AKI as the outcome variable. The proportional odds model accommodated the ordinal nature of the multilevel response variable AKI (i.e., “no AKI” better than “stage I AKI” better than “stage II AKI” better than “stage III AKI”). The resulting odds ratio estimated the relative odds of developing a more serious level of AKI in patients given colloids versus patients given crystalloids. The proportional odds assumption was verified graphically. Within our proportional odds model, we adjusted for intraoperative hypotension, duration of surgery, intraoperative urine output, and estimated blood loss.
Various sensitivity analyses that explored the effects of changes to the causal assumptions of intraoperative variables on the estimated relationship between colloid use and AKI were performed, specifically using transfusions and vasopressors as confounders rather than mediators. We also conducted a sensitivity analysis using multivariable regression adjustment among all 44,598 patients meeting inclusion and exclusion criteria to evaluate whether the conclusions with this approach differed from those drawn from our propensity-matched sample. Furthermore, we carried out the sensitivity analysis based on two groups of patients matched on all considered potential confounders including intraoperative blood loss, hypotension, urine output, and duration of surgery. And finally, we conducted a sensitivity analysis that explored the association between colloid use and AKI when postoperative assessment of renal function was based on the serum creatinine measurement only within 48 postoperative hours.
Given total sample size of 29,360 and 4.0% of patients given noncolloids experienced AKI, we had approximately 90% power at the 0.025 significance level to detect an odds ratio of 1.22 or greater comparing patients given colloids to patients receiving only crystalloids during the surgery.
Secondary Outcomes
To evaluate the relationship between volume of colloids and AKI, we used all (prematched) patients who were given colloids. A separate proportional odds model was developed to adjust for all baseline and intraoperative potential confounding variables listed in the primary analysis. We performed sensitivity analyses exploring whether the estimated relationship between volume of colloids and AKI changes based on assumptions on intraoperative confounders.
Because the effect of colloids on postoperative kidney function might depend on baseline risk of renal injury, we evaluated the interaction between colloid use and the Kheterpal risk index for AKI26 within the primary analysis proportional odds model. A significant interaction would suggest that the relationship between colloid use and AKI varies as a function of baseline risk of AKI. Kheterpal classes 4 and 5 were combined because there were few class 5 patients.
We also assessed the association between colloid use and inhospital mortality using the matched patients. A logistic regression model was developed to account for the intraoperative potential confounding variables listed in the primary analysis.
Finally, we assessed the association between colloid use and 90-day mortality. Because we only had complete data on mortality through November 1, 2011, we used the subset of matched pairs having both surgeries before August 2, 2011. A Cox proportional hazards regression model27 was used to assess the hazard ratio of dying within 90 days of the surgery between patients receiving colloids and crystalloids. Patients without recorded death before November 1, 2011 were censored on that date. The Cox model accounted for the intraoperative potential confounding variables listed in the primary analysis. Given an incidence of mortality as low as 3.44% for the crystalloid group and sample size of 23,630 patients, the study had 90% power to detect a 31% or greater possible difference in 90-day mortality (i.e., hazard ratio of 1.31 or more) at the 0.006 significance level.
Results
We obtained data for 44,176 surgeries, including 22,289 (50.4%) who received colloid and crystalloids and 21,887 (49.6%) who received only crystalloids as a part of fluid administration. Then, based on patients’ baseline characteristics, we successfully 1:1 matched 14,680 patients given colloids (66% of the total patients given colloid) to control patients given only crystalloids for a total of 29,360 patients.
As seen in the left panel of table 1, the two groups of patients were imbalanced on 15 of 26 baseline potential confounders (ASD >5%) before matching; however, all patient baseline variables were well balanced after matching (table 1). The top 15 major diagnostic or therapeutic surgical procedures (78% of the total) are shown in table 1. The median [first quartile, third quartile] amount of colloid given to the matched colloid group was 500 [100, 500] ml along with 2,800 [2,000, 3,800] ml of crystalloid; for crystalloid group, the amount of crystalloid given was 2,200 [1,600, 2,900] ml (table 2).
Table 2.
Results for the Primary Hypothesis and First Secondary Hypothesis
| N (%) of Patients |
|||
|---|---|---|---|
| Colloids | Noncolloids | Adjusted Odds Ratio (97.5% CI) | |
| All patients | 1.21 (1.06–1.38) | ||
| No AKI | 13,802 (94.0) | 14,074 (95.9) | |
| Stage I AKI | 635 (4.3) | 447 (3.0) | |
| Stage II AKI | 159 (1.1) | 112 (0.8) | |
| Stage III AKI | 84 (0.6) | 47 (0.3) | |
| Kheterpal Class I* | |||
| No AKI | 8,834 (96.6) | 8,994 (97.8) | |
| Stage I AKI | 228 (2.5) | 150 (1.6) | |
| Stage II AKI | 48 (0.5) | 33 (0.4) | |
| Stage III AKI | 32 (0.4) | 17 (0.2) | |
| Kheterpal Class II* | |||
| No AKI | 3,046 (92.8) | 3,101 (94.8) | |
| Stage I AKI | 172 (5.2) | 130 (4.0) | |
| Stage II AKI | 43 (1.3) | 29 (0.9) | |
| Stage III AKI | 23 (0.7) | 10 (0.3) | |
| Kheterpal Class III* | |||
| No AKI | 1,419 (87.5) | 1,490 (91.6) | |
| Stage I AKI | 136 (8.4) | 98 (6.0) | |
| Stage II AKI | 47 (2.9) | 29 (1.8) | |
| Stage III AKI | 20 (1.2) | 9 (0.6) | |
| Kheterpal Class IV/V* | |||
| No AKI | 503 (79.6) | 539 (84.2) | |
| Stage I AKI | 99 (15.7) | 69 (10.8) | |
| Stage II AKI | 21 (3.3) | 21 (3.3) | |
| Stage III AKI | 9 (1.4) | 11 (1.7) | |
The adjusted odds ratio is reported as colloid vs. noncolloid use, for matched samples, adjusted for intraoperative hypotension, duration of surgery, urine output, and blood loss using proportional odds model. The Wald test P value for effect of colloid use is 0.001. The Wald test P value for interaction of colloid use and baseline Kheterpal index is 0.84, implying a consistent effect of colloid use across all Kheterpal risk classes.
Kheterpal risk index for AKI characterizes baseline risk of AKI.
AKI = acute kidney injury.
Primary Outcome
Results for the primary multilevel AKI outcome are summarized in table 2. Using the matched patients, there was a significant association between colloid use and increased level of AKI after adjusting for the intraoperative potential confounding variables (P = 0.001), with adjusted proportional odds ratio (97.5% CI) comparing the colloid and crystalloid groups of 1.21 (1.06 to 1.38). In other words, after controlling for the aforementioned potential confounding variables, the odds of developing a more serious level of AKI for patients given both crystalloids and colloids were 21% (6 to 38%) greater than in patients given only crystalloids.
Results of various sensitivity analyses—which explored multivariable regression adjustment instead of matching, matching on all variables, and various modifications to the assumptions regarding whether an intraoperative variable was a confounder or not—were consistent with the results on the primary model showing a significant association between HES and AKI. Only the two models when intraoperative blood transfusion was considered as confounder did not show a significant association (table 3). Furthermore, the estimated colloid use effect was similar to one claimed in the primary analysis when the renal function assessment was based on 48 postoperative hours (as in the original Acute Kidney Injury Network formulation) instead of 7 days.
Table 3.
Sensitivity Analysis on Primary Hypothesis
| Considered Potential Confounders for Adjustment |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | Sample | Baseline | Estimated Blood Loss | Hypotension | Duration of Surgery | Urine Output | Transfusion | Vasopressor Use | Adjusted Odds Ratio (97.5% CI) |
P Value∥ |
| Primary model* | Matched patients | • | • | • | • | 1.21 (1.06–1.38) | <0.001 | |||
| Sensitivity analysis | ||||||||||
| Model included all patients† | All patients | • | • | • | • | • | 1.26 (1.12–1.42) | <0.001 | ||
| Model on patients matched on all potential confounders‡ |
Matched patients | 1.22 (1.07–1.40) | 0.001 | |||||||
| Treating transfusion as potential confounders* |
Matched patients | • | • | • | • | • | 1.13 (0.99–1.29) | 0.04 | ||
| Treating vasopressor use as potential confounders* |
Matched patients | • | • | • | • | • | 1.16 (1.02–1.32) | 0.011 | ||
| Treating transfusion and vasopressor use as potential confounders* |
Matched patients | • | • | • | • | • | • | 1.10 (0.96–1.25) | 0.12 | |
| Treating as duration of surgery as a mediator*§ |
Matched patients | • | • | • | 1.35 (1.19–1.53 | <0.001 | ||||
| Treating as intraoperative hypotension as a mediator*§ |
Matched patients | • | • | • | 1.28 (1.13–1.46) | <0.001 | ||||
| Treating as intraoperative blood loss as a mediator*§ |
Matched patients | • | • | • | 1.20 (1.06–1.37) | 0.001 | ||||
Explored models showed association between colloid use and increased odds of AKI (P < 0.025), except for the models when intraoperative blood transfusion was considered as a confounder.
Multivariable proportional odds model adjusts for marked intraoperative potential confounders among 29,360 matched patients. Matching was based on patient’s baseline characteristics listed in the table 1.
This multivariable regression model adjusts for baseline characteristics and intraoperative potential confounders among all 44,176 patients meeting inclusion and exclusion criteria. Matching was based on patients’ baseline characteristics listed in the table 1.
This model based on 24,542 matched patients. Matching was based on patient baseline and intraoperative potential confounders (excluding transfusion and vasopressor) listed in the table 1.
Exploring the results if our assumptions regarding confounding were untrue. That is, the variable might be potential mediator and need to be excluded from the adjustment.
A significance criterion is 0.025.
AKI = acute kidney injury.
Secondary Outcomes
Using all 22,289 (prematched) patients who were given colloids and adjusting for all baseline and intraoperative potential confounding variables, we found a significant association between the total volume of colloid given and risk of developing more advanced stage of AKI (Wald test P < 0.001; a significance criterion of 0.025/4 = 0.006). The corresponding adjusted odds ratio (Bonferroni-adjusted 97.5% CI) was 1.44 (1.26 to 1.64); that is, for each additional unit (500 ml) of colloids given, the odds of developing a more serious stage of AKI increased by an estimated 44% (26 to 64%). The relationship between colloid volume and the probability of developing AKI is showed in figure 3. Results of sensitivity analyses that explored various assumptions regarding the intraoperative confounders were highly consistent with the results of the primary model (table 4).
Fig. 3.
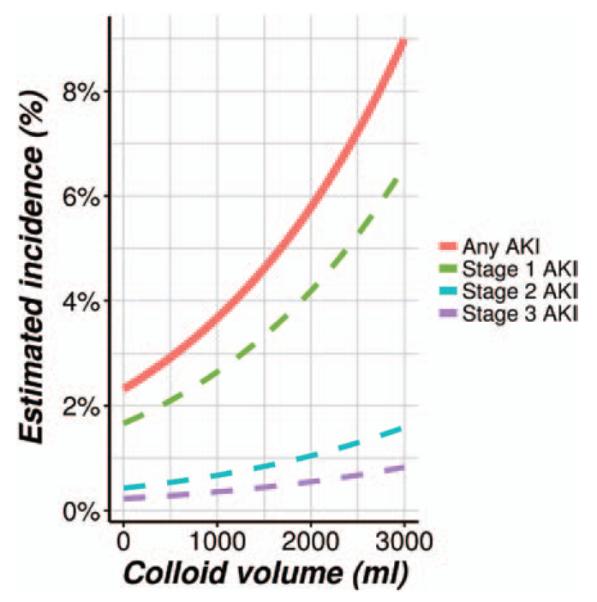
Relationship between hydroxyethyl starch volume and probability of acute kidney injury (AKI).
Table 4.
Sensitivity Analysis on Dose-dependency Hypothesis
| Considered Potential Confounders for Adjustment |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Baseline | Estimated Blood Loss | Hypotension | Duration of Surgery | Urine Output | Transfusion | Vasopressor Use | Adjusted Odds Ratio (97.5% CI) |
P Value† |
| Primary model* | • | • | • | • | • | 1.44 (1.26–1.64) | <0.001 | ||
| Sensitivity analysis | |||||||||
| Treating transfusion as potential confounders* |
• | • | • | • | • | • | 1.38 (1.21–1.58) | <0.001 | |
| Treating vasopressor use as potential confounders* |
• | • | • | • | • | • | 1.44 (1.26–1.65) | <0.001 | |
| Treating transfusion and vasopressor use as potential confounders* |
• | • | • | • | • | • | • | 1.39 (1.21–1.59) | <0.001 |
We used all 22,289 (prematched) patients who were given colloids for all models listed above. All explored models showed association between volume of colloid and increased odds of acute kidney injury (P < 0.006).
Multivariable proportional odds model adjusts for all patient baseline (table 1) and marked intraoperative potential confounders among 22,289 patients who were given colloid.
A significance criterion is 0.006 for the secondary hypothesis.
There was no association between in-hospital mortality and colloid use (P = 0.81 using a significance criterion of 0.025/4 = 0.006), with an adjusted odds ratio (Bonferroni-adjusted 97.5% CI) of 0.97 (0.69 to 1.36) comparing colloids with crystalloids. The number (percent) of in-hospital mortalities was 165 (1.12%) for colloid group patients and 137 (0.93%) for crystalloid. Colloid use was not associated with 90-day mortality using the available 11,815 matched pairs (81% of total matched patients), with adjusted hazard ratio (Bonferroni-adjusted 97.5% CI) of 0.84 (0.68 to 1.03), P = 0.02 (insignificant after Bonferroni correction because P < 0.025/4 = 0.006). The number (percent) of 90-day mortality was 370 (3.13%) and 407 (3.44%) for colloid and crystalloid groups (fig. 4).
Fig. 4.
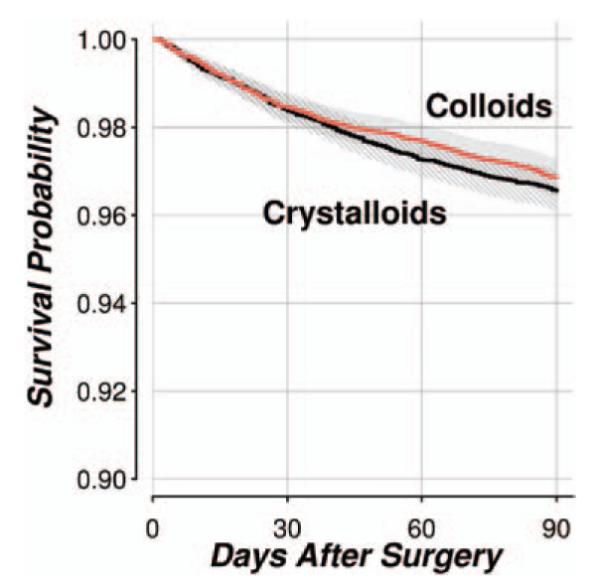
Kaplan–Meier survival curves comparing 90-day survival probabilities in the hydroxyethyl starch and crystalloid groups.
We did not find a significant interaction between baseline risk and colloid use (Wald test P = 0.84; a significance criterion of 0.025/4 = 0.006); that is, the odds of developing more progressive stages of AKI with colloid use did not differ significantly across the Kheterpal AKI risk classes (table 2).
Discussion
Although the exact mechanism remains unclear, the potential for colloids to cause kidney injury is well known. Morphological changes caused by tubular swelling due to cytoplasmic vacuole formation is termed osmotic nephrosis and is thought to be among the causes of renal toxicity induced by HES.28,29 The oncotic force of these solutions may also impair renal function by decreasing filtration pressure.28 And finally, high-molecular-weight HES has also been associated with a higher degree of accumulation due to lower degradability in the interstitial space and the reticulo-endothelial system, factors probably contributing to toxicity and other side effects including pruritus.30
Our results support these concerns: we found that intraoperative HES use was significantly associated with a 21% increased risk of AKI. Furthermore, the risk of developing AKI increased as a function of colloid volume, indicating that harm was dose dependent as one might expect from a drug-related toxicity. In contrast, mortality was not worse in patients given HES—although there is a well-established relationship between AKI and increased mortality.31 It is likely that high-molecular-weight starches worsen mortality as well as kidney function, but the mortality effect was obscured by the many other factors that contribute to postoperative death.
Patients at high risk of developing AKI, unsurprisingly, had worse postoperative kidney function. However, patients with higher baseline risk of postoperative kidney injury did not disproportionately develop a more severe stage of AKI in response to colloid use than those with fewer risk factors. Thus, independent of the patients’ preoperative risk for renal injury, the adjusted odds ratios of developing AKI was similar among various baseline risk classes.
In two recent large randomized trials, a newer low-molecular-weight HES (130/0.42) was compared with Ringer’s acetate in patients with sepsis18 and with saline in intensive care patients.19 In each, patients given HES were more likely to require renal replacement therapy, and in the former, there was also increased 90-day mortality. There are no comparable large randomized, controlled trials in the surgical patient population evaluating the renal effects of different types of plasma expanders. However, our retrospective results suggest that high-molecular-weight starches are associated with increased morbidity in surgical patients
In observational cohort studies, it is sometimes unclear whether or not to treat a particular variable as confounding of the effect of interest or to treat it as a potential mechanism by which the exposure might affect the outcome. Commonly, a “kitchen-sink” approach is used whereby all variables are treated as potential confounders. This risks over-adjustment or “adjusting away” the effect of interest. In our primary analysis, we thus adjusted only for variables meeting the definition of potential confounders (i.e., those assumed to both directly affect the use of colloids and affect the risk of AKI) and excluded others that appeared to be potential mediator variables which are actually caused by the exposure of interest.
Because it can be difficult to determine whether a particular variable is a confounder or mediator, we conducted various sensitivity analyses. For example, we assumed that intraoperative estimated blood loss, intraoperative hypotension, urine output, and duration of surgery were potential confounders and adjusted for each factor in all analyses. In contrast, intraoperative vasopressor use (use of either of dobutamine, dopamine, epinephrine, norepinephrine, phenylephrine, or vasopressin) and intraoperative blood product transfusion (erythrocyte transfusion) may be associated with the AKI outcome1 but were not considered to be confounders in the primary analysis. This was based on our assumption and clinical judgment that administration of HES is mainly triggered by blood loss and that administration of transfusions and vasopressors happens thereafter and thus might not influence the decision to administer colloids. Thus, vasopressor use and blood transfusions might be mediators. We therefore did not adjust for these two variables in the primary analyses. It sometimes is very difficult to make a clear distinction between confounders and mediators and thus we added several sensitivity analyses, in which vasopressor usage and/or transfusion were used as confounders. The results of each sensitivity analysis supported our primary analysis, suggesting that the result is robust. Specifically, point estimates of treatment odds ratios over a wide range of assumptions which ranged from 1.10 to 1.35 were all close to the estimate from our primary analysis which was 1.21. Furthermore, all but two approaches continued to demonstrate a statistically significant worsening with colloid administration. Taken together, the results indicate a clinically important and statistically significant association between HES administration and worsened outcomes over a wide range of assumptions.
The potential confounders we considered included a validated set of weighted independent predictors.26 These factors and others we thought important were included in our analysis through propensity matching or multivariable adjustment. But undoubtedly, there are other preoperative and intraoperative variables that increase the risk of developing postoperative AKI. As with any statistical analysis, our ability to adjust for potential confounding is limited to available data. Our Perioperative Health Documentation System includes pre-, intra-, and postoperative data and we are fairly confident that these data are as complete as possible. Furthermore, the amount of missing data was minimal. However, the extent to which unobserved variables contributed cannot be determined and limits our ability to make causal conclusions, which are most reliably derived from randomized trials. Consequently, the associations we report should not be considered evidence of a causal relationship. Removing patients with missing potential covariates might also alternate the results. And last but not least our results can only be applied to adult patients undergoing noncardiac surgical procedures.
In summary, there was a statistically significant and clinically important dose-dependent association between intraoperative Hextend administration and postoperative kidney injury in adult patients having major noncardiac surgery. Our results are consistent with randomized trials in critical care patients. We evaluated a high-molecular-weight colloid solution.
What We Already Know about This Topic
Hydroxyethyl starch increases the risk of acute kidney injury and mortality in critically ill patients, but whether its intraoperative use increases acute kidney injury remains unknown
What This Article Tells Us That Is New
Using a large database (44,176 adult patients undergoing noncardiac surgery), a dose-dependent renal toxicity of Hextend (high-molecular-weight hydroxyethyl starch) was observed: odds ratio to develop a more serious level of acute kidney injury than crystalloids was 21% (95% CI, 6 to 38%, P < 0.001)
Acknowledgments
Dr. Dalton’s effort was supported by the Clinical and Translational Science Collaborative of Cleveland, grant no. KL2TR000440 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health (NIH) and NIH roadmap for Medical Research (Bethesda, Maryland). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. All other funding was provided by institutional and/or departmental sources.
Footnotes
Competing Interests The Department of Outcomes Research receives funding from Hospira (Lake Forest, Illinois) and Fresenius (Bad Homburg, Germany) (the makers of Voluven) for other projects. None of the investigators has a personal financial interest related to this research.
on the world wide web: www.or.org. Information on purchasing reprints may be found at www.anesthesiology.org or on the masthead page at the beginning of this issue. Anesthesiology’s articles are made freely accessible to all readers, for personal use only, 6 months from the cover date of the issue.
HCUP CCS. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD. Available at: www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed February 20, 2013.
References
- 1.Kheterpal S, Tremper KK, Englesbe MJ, O’Reilly M, Shanks AM, Fetterman DM, Rosenberg AL, Swartz RD. Predictors of postoperative acute renal failure after noncardiac surgery in patients with previously normal renal function. Anesthesiology. 2007;107:892–902. doi: 10.1097/01.anes.0000290588.29668.38. [DOI] [PubMed] [Google Scholar]
- 2.Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, Cywinski J, Thabane L, Sessler DI. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: Toward an empirical definition of hypotension. Anesthesiology. 2013;119:507–15. doi: 10.1097/ALN.0b013e3182a10e26. [DOI] [PubMed] [Google Scholar]
- 3.Hanart C, Khalife M, De Villé A, Otte F, De Hert S, Van der Linden P. Perioperative volume replacement in children undergoing cardiac surgery: Albumin versus hydroxyethyl starch 130/0.4. Crit Care Med. 2009;37:696–701. doi: 10.1097/CCM.0b013e3181958c81. [DOI] [PubMed] [Google Scholar]
- 4.Guidet B, Mosqueda GJ, Priol G, Aegerter P. The COASST study: Cost-effectiveness of albumin in severe sepsis and septic shock. J Crit Care. 2007;22:197–203. doi: 10.1016/j.jcrc.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Sinclair S, James S, Singer M. Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: Randomised controlled trial. BMJ. 1997;315:909–12. doi: 10.1136/bmj.315.7113.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gravlee GP, Brockschmidt JK. Accuracy of four indirect methods of blood pressure measurement, with hemodynamic correlations. J Clin Monit. 1990;6:284–98. doi: 10.1007/BF02842488. [DOI] [PubMed] [Google Scholar]
- 7.Stewart JA. Adding insult to injury: Care of patients with acute kidney injury. Br J Hosp Med (Lond) 2009;70:372–3. doi: 10.12968/hmed.2009.70.7.43116. [DOI] [PubMed] [Google Scholar]
- 8.Uchino S, Bellomo R, Bagshaw SM, Goldsmith D. Transient azotaemia is associated with a high risk of death in hospitalized patients. Nephrol Dial Transplant. 2010;25:1833–9. doi: 10.1093/ndt/gfp624. [DOI] [PubMed] [Google Scholar]
- 9.Bihorac A, Yavas S, Subbiah S, Hobson CE, Schold JD, Gabrielli A, Layon AJ, Segal MS. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–8. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- 10.Prowle JR, Bellomo R. Fluid administration and the kidney. Curr Opin Crit Care. 2013;19:308–14. doi: 10.1097/MCC.0b013e3283632e29. [DOI] [PubMed] [Google Scholar]
- 11.Shoemaker WC, Montgomery ES, Kaplan E, Elwyn DH. Physiologic patterns in surviving and nonsurviving shock patients. Use of sequential cardiorespiratory variables in defining criteria for therapeutic goals and early warning of death. Arch Surg. 1973;106:630–6. doi: 10.1001/archsurg.1973.01350170004003. [DOI] [PubMed] [Google Scholar]
- 12.Holte K, Sharrock NE, Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth. 2002;89:622–32. doi: 10.1093/bja/aef220. [DOI] [PubMed] [Google Scholar]
- 13.Arieff AI. Fatal postoperative pulmonary edema: Pathogenesis and literature review. Chest. 1999;115:1371–7. doi: 10.1378/chest.115.5.1371. [DOI] [PubMed] [Google Scholar]
- 14.Prien T, Backhaus N, Pelster F, Pircher W, Bünte H, Lawin P. Effect of intraoperative fluid administration and colloid osmotic pressure on the formation of intestinal edema during gastrointestinal surgery. J Clin Anesth. 1990;2:317–23. doi: 10.1016/0952-8180(90)90077-g. [DOI] [PubMed] [Google Scholar]
- 15.Wilson J, Woods I, Fawcett J, Whall R, Dibb W, Morris C, McManus E. Reducing the risk of major elective surgery: Randomised controlled trial of preoperative optimisation of oxygen delivery. BMJ. 1999;318:1099–103. doi: 10.1136/bmj.318.7191.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mythen MG, Webb AR. Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch Surg. 1995;130:423–9. doi: 10.1001/archsurg.1995.01430040085019. [DOI] [PubMed] [Google Scholar]
- 17.Gan TJ, Soppitt A, Maroof M, el-Moalem H, Robertson KM, Moretti E, Dwane P, Glass PS. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology. 2002;97:820–6. doi: 10.1097/00000542-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Aneman A, Madsen KR, Moller MH, Elkjaer JM, Poulsen LM, Bendtsen A, Winding R, Steensen M, Berezowicz P, Soe-Jensen P, Bestle M, Strand K, Wiis J, White JO, Thornberg KJ, Quist L, Nielsen J, Andersen LH, Holst LB, Thormar K, Kjaeldgaard AL, Fabritius ML, Mondrup F, Pott FC, Moller TP, Winkel P, Wetterslev J. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367:124–34. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- 19.Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, Glass P, Lipman J, Liu B, McArthur C, McGuinness S, Rajbhandari D, Taylor CB, Webb SA. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–11. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 20.Patel A, Waheed U, Brett SJ. Randomised trials of 6% tetra-starch (hydroxyethyl starch 130/0.4 or 0.42) for severe sepsis reporting mortality: Systematic review and meta-analysis. Intensive Care Med. 2013;39:811–22. doi: 10.1007/s00134-013-2863-6. [DOI] [PubMed] [Google Scholar]
- 21.Zarychanski R, Abou-Setta AM, Turgeon AF, Houston BL, McIntyre L, Marshall JC, Fergusson DA. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: A systematic review and meta-analysis. JAMA. 2013;309:678–88. doi: 10.1001/jama.2013.430. [DOI] [PubMed] [Google Scholar]
- 22.Gattas DJ, Dan A, Myburgh J, Billot L, Lo S, Finfer S. Fluid resuscitation with 6 % hydroxyethyl starch (130/0.4 and 130/0.42) in acutely ill patients: Systematic review of effects on mortality and treatment with renal replacement therapy. Intensive Care Med. 2013;39:558–68. doi: 10.1007/s00134-013-2840-0. [DOI] [PubMed] [Google Scholar]
- 23.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 25.Austin PC. Some methods of propensity-score matching had superior performance to others: Results of an empirical investigation and Monte Carlo simulations. Biom J. 2009;51:171–84. doi: 10.1002/bimj.200810488. [DOI] [PubMed] [Google Scholar]
- 26.Kheterpal S, Tremper KK, Heung M, Rosenberg AL, Englesbe M, Shanks AM, Campbell DA., Jr Development and validation of an acute kidney injury risk index for patients undergoing general surgery: Results from a national data set. Anesthesiology. 2009;110:505–15. doi: 10.1097/ALN.0b013e3181979440. [DOI] [PubMed] [Google Scholar]
- 27.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 28.Schortgen F, Brochard L. Colloid-induced kidney injury: Experimental evidence may help to understand mechanisms. Crit Care. 2009;13:130. doi: 10.1186/cc7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw AD, Kellum JA. The risk of AKI in patients treated with intravenous solutions containing hydroxyethyl starch. Clin J Am Soc Nephrol. 2013;8:497–503. doi: 10.2215/CJN.10921012. [DOI] [PubMed] [Google Scholar]
- 30.Mitra S, Khandelwal P. Are all colloids same? How to select the right colloid? Indian J Anaesth. 2009;53:592–607. [PMC free article] [PubMed] [Google Scholar]
- 31.Moore EM, Bellomo R, Nichol AD. The meaning of acute kidney injury and its relevance to intensive care and anaesthesia. Anaesth Intensive Care. 2012;40:929–48. doi: 10.1177/0310057X1204000604. [DOI] [PubMed] [Google Scholar]