Figure 8.
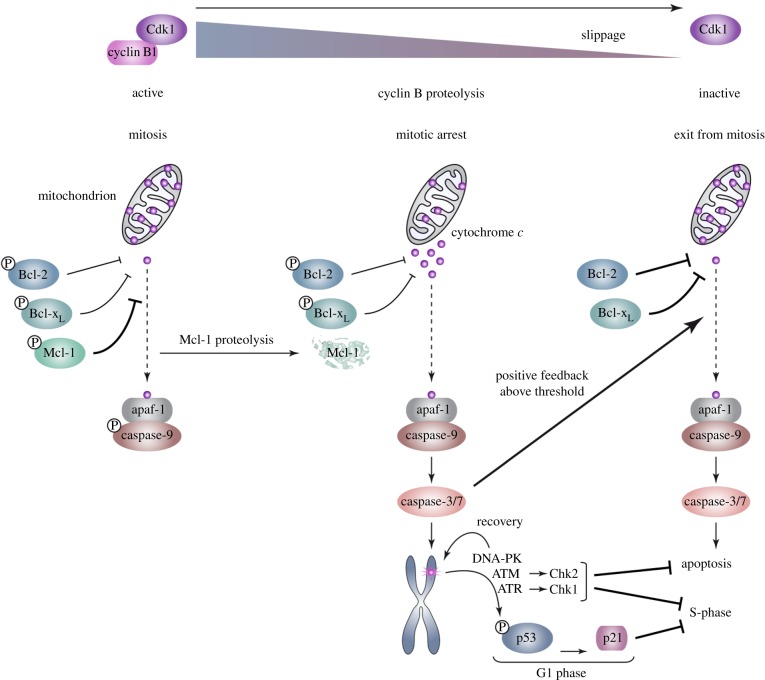
Cellular responses to microtubule poisons are determined by caspase-dependent DNA damage signalling initiated during delayed mitosis. During normal mitosis, release of cytochrome c from mitochondria is inhibited by the action of Bcl-2, Bcl-xL and Mcl-1; downstream caspase activation is restrained by the inhibitory phosphorylation of caspase-9. Increasing phosphorylation of Bcl-2 and Bcl-xL, however, reduces their activity, whereas phosphorylation of Mcl-1 by CDK1–cyclin B initiates its proteolytic destruction. After a prolonged mitotic arrest, this results in the partial release of cytochrome c from mitochondria and the subapoptotic activation of caspase-3/7 when coupled with a slow decline in CDK1–cyclin B kinase activity and dephosphorylation of caspase-9. Caspase-3/7 activity results in localized DNA damage and activation of ATM, ATR and DNA-PK. The mitotic DNA damage response is amplified as cells slip out of mitosis, resulting in the phosphorylation of p53 and induction of p21, which inhibits CDK activity required for S-phase. ATM and ATR also activate Chk2 and Chk1, respectively, which inhibit subsequent cell cycle progression. ATM, ATR and DNA-PK also have functions in recovery from telomere damage and restrain apoptosis.