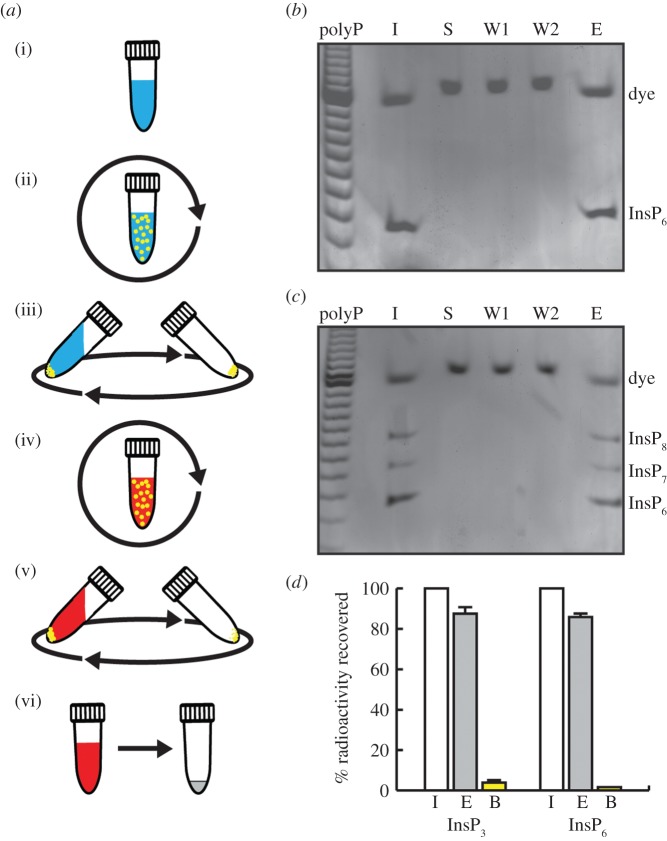
Figure 1.
TiO2 purifies inositol phosphates. (a) Flowchart describing the five-step TiO2 bead extraction procedure. (i) Acidic solution (blue) containing inositol phosphates is incubated with (ii) TiO2 beads (yellow) for 10 min, before (iii) spinning and washing the beads twice with 1 M PA. Elution occurs by incubation (iv) at basic pH (red) with subsequent spinning and recovering the supernatant (v). This is evaporated (vi) to concentrate and neutralize (grey) the extract. (b) InsP6 diluted in 1 M PA was purified using TiO2 and subjected to PAGE with toluidine blue staining. I, input; S, supernatant; W1 and W2, washes; E, eluted. While all the eluted InsP6 was loaded on the gel only 1/10 (approx. 100 µl) of the S, W1 and W2 fractions were loaded. The acid in these fractions results in slightly compressed and slower migration of the orange G dye. (c) As (b), but using a PA extraction from vegetative state D. discoideum cells as input. These toluidine blue-stained gels are representative of experiments performed at least three times. (d) To calculate the exact percentage of recovery, radioactive 3H-Ins(1,4,5)P3 and 3H-InsP6 were subjected to TiO2 purification. The radioactivity recovered (E) and radioactivity remaining on TiO2 beads (B) were normalized to the respective radioactive input (I). The graph showing the average ± s.d. (n = 4) is representative of two independent experiments with matching results.