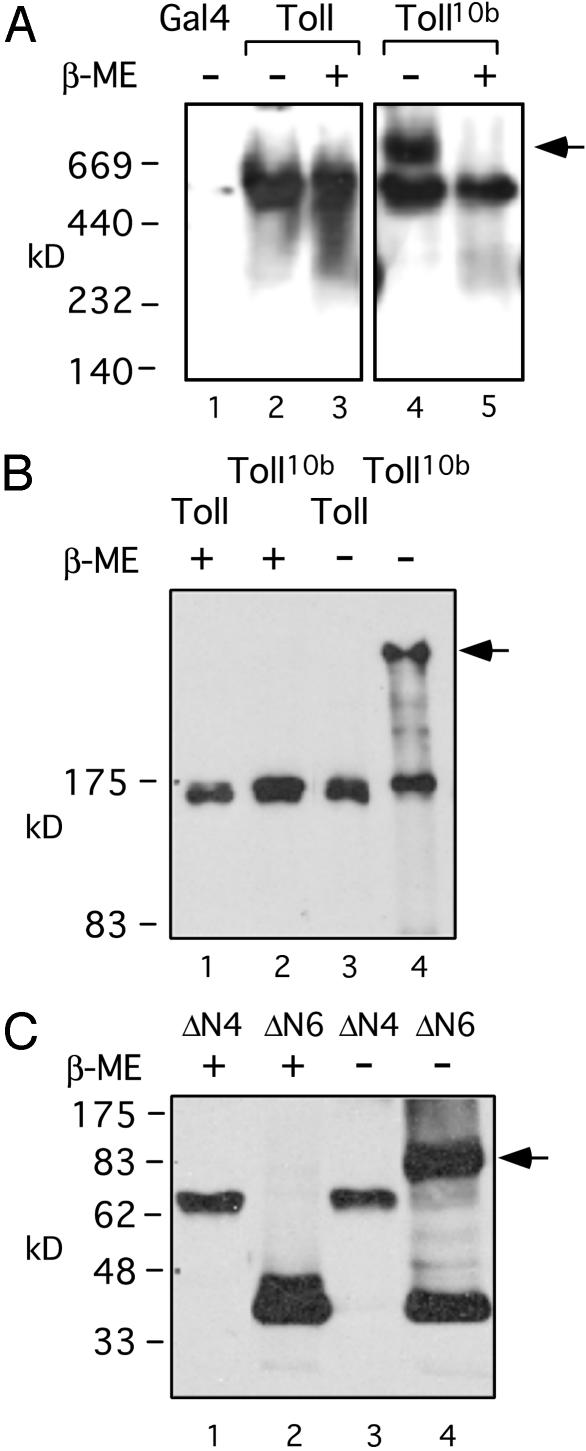
Fig. 3.
Disulfide linkages in constitutively active Toll complexes. (A) Western blot of Myc-tagged protein extracts from various fly strains in native gel. The Toll or Toll10b protein extracts from adult female flies, as well as Gal4 fly extracts (lane 1), were separated on a 4–15% gradient native acrylamide gel (Bio-Rad). Some samples were treated with β-ME as indicated before loading onto the gel. The arrow indicates a complex of ≈750 kDa in the Toll10b extract (lane 4). After β-ME treatment, both Toll and Toll10b behaved as ≈500-kDa proteins. We cannot determine whether these proteins represent monomers, dimers, or bigger complexes, because the apparent size of a protein on native gel depends on many factors, such as charge, shape, and other associated proteins. (B) Western blot of transgenic fly extracts in denaturing gel. The transgenic fly extracts, with or without β-ME treatment as indicated, were analyzed. Only Toll10b forms a complex under nonreducing condition, and the size indicates a probable homodimer (lane 4, arrow). There is no commercially available high-molecular-weight marker at this range for denaturing gels, and we were not able to calculate the size of the complex more accurately. (C) Western blot of transfected S2 cells extracts. The extracts were analyzed by using denaturing SDS gels and Western blotting, and by using anti-V5 antibodies. Only the ΔN6, not the inactive ΔN4, forms a disulfide-linked complex. The size indicates that it is probably a homodimer (lane 4, arrow).