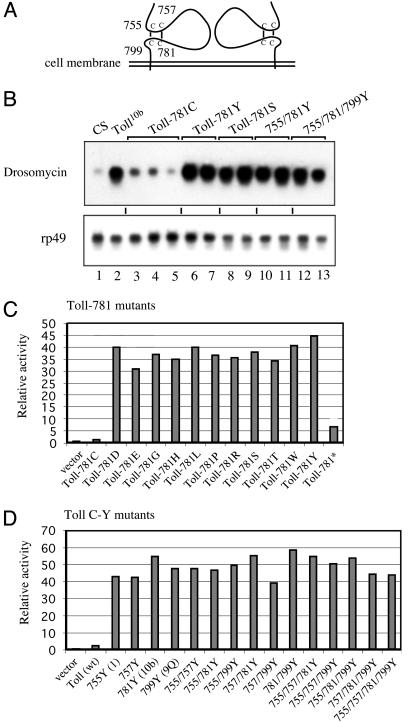
Fig. 5.
The cysteine-containing motif as an autoinhibitory module. (A) Cysteine-containing motifs of a Toll dimer. The numbering of the cysteine residues is indicated. The two disulfide bonds are speculative, but possible, according to the structure of a related protein, glycoprotein 1bα (28, 38, 39). (B) Northern blot of mutants of the cysteine-containing motif. The Toll-781C is wild type, and other amino acid substitutions are as indicated. The proteins were expressed in transgenic flies by using the YP1-Gal4-UAS system. The expression of drosomycin and rp49 was analyzed by Northern blotting. Three independent 781C lines (lanes 3–5) and two independent lines of each of the other mutants were assayed (lanes 6–13). The CS and Toll10b flies were included for comparison (lanes 1 and 2, respectively). (C) Transfection assays of cysteine-781 mutants. The cysteine-781 was changed to other amino acids as indicated, and the activities of the mutants were assayed by cotransfection with a drosomycin-luciferase reporter in S2 cells. The Toll-781* contains a stop codon and, therefore, should produce a truncated protein. (D) Transfection assays of single or multiple cysteine-to-tyrosine mutants. Single or multiple cysteine-to-tyrosine changes were introduced into the wild-type Toll, and the activities were assayed by transfection as described for C. The mutations of the four cysteines in any combination, including quadruple mutation, have the same result of constitutive receptor activation. Overall, these data show that the cysteine-containing motif is an autoinhibitory module.