Abstract
Rabies virus (RV) nucleoprotein (N) tightly encapsidates the genomic and antigenomic RNA of RV to form the viral ribonucleoprotein (RNP) complex. Antigens, such as N, presented in a highly organized structure are sufficient and even desirable to activate B cells to proliferate and produce antibodies. In addition to activating B cells to proliferate, it has been shown that RV N in the RNP complex induces potent T helper cell responses resulting in long-lasting and strong humoral immune responses against RV. The possibility to systematically incorporate foreign genes into the genome of RV and produce a recombinant virus allows us to examine whether the immunogenicity of foreign antigens can be enhanced by incorporation into the RV RNP structure. To test this hypothesis we constructed a recombinant RV expressing a RV N-GFP fusion protein. The chimeric N-GFP fusion protein was efficiently expressed and incorporated into RV RNP and virions. Moreover, the recombinant RNP induces a strong humoral immune response against GFP in mice. In contrast, mice inoculated with GFP alone or a combination of wild-type RV RNPs and GFP did not trigger any GFP-specific humoral responses using the same immunization schedule. These data indicate the usefulness of RV-based vectors as killed vaccines against other infectious diseases.
Keywords: nucleocapsid, fusion protein, vaccine
Rabies virus (RV) is a nonsegmented negative-strand RNA virus within the Rhabdoviridae family (1). The RV genome is ≈12 kb in size and encodes five monocistronic mRNAs encoding the nucleocapsid protein (N), phosphoprotein (P), matrix protein (M), the transmembrane glycoprotein (G), and the viral RNA-dependent RNA polymerase (L) (2). The N efficiently and specifically encapsidates the viral RNA to form the ribonucleoprotein (RNP) complex, which provides the template for RNA transcription and replication by the viral polymerase complex, which includes the P and L (3, 4). The RV M bridges the RNP with the cytoplasmic domain of RV G, which is embedded in the host cell-derived viral membrane (5). The extracellular domain of the RV G mediates infection of the host cell (6).
Recombinant live-viral vectors expressing foreign antigens efficiently induce potent cellular and humoral immune responses against the expressed antigens. The possibility that RV can be used as an expression vector was shown earlier with the model gene chloramphenicol acetyltransferase, and the results from these studies indicated that foreign genes can be expressed stably in RV (7). The possibility that RV could be used as an HIV-1 vaccine was tested, and it was shown in several studies that the expression of HIV-1 Env or Gag resulted in potent immune responses directed against HIV-1 (8–10). In addition, we demonstrated that RV vaccine vectors tolerate large, multiple foreign genes, up to 6.5 kb, and that live-viral RV vectors with a very low pathogenic potential can be constructed (11). Moreover, our results have indicated that killed RV virions containing foreign glycoproteins such as hepatitis C E2 or HIV-1 gp160 induce strong B cell responses in a small animal model (12, 13).
Part of the immune protection from pathogens requires at least a strong B cell-mediated humoral response. It has been previously shown with different virus-based expression systems that a variety of viral particles or virus-like particles can serve as carriers of foreign B cell epitopes within the viral capsid or envelope protein of the viral particle. These epitope carriers resulted in strong B cell responses against the expressed foreign epitopes indicating a usefulness of carrier proteins (14–20). However, most of these approaches are restricted to incorporation of only short peptides that define epitopes due to the restricted cloning capacity within the carrier proteins. We hypothesized that RV N as a carrier protein for foreign epitopes might circumvent this problem. A large body of evidence suggests that the following features, or a combination of them, are particularly important to induce strong humoral responses against certain epitopes or antigens. (i) Epitopes or antigens presented in the context of a tightly, highly organized structure activate B cells to proliferate and induce IgM production (21, 22). (ii) A strong T cell antigen linked to a B cell epitope enhances the B cell-specific immune response. Apropos this study, the RV N is able to prime T cells and induce RV N-specific antibodies (23, 24). (iii) Because antigen-presenting cells play an important role in the development of immunity, the strong humoral immune response against RV N suggests that RV N must be efficiently presented by antigen-presenting cells (24). In addition, the stability of the antigen can be important. RV RNP is highly resistant to proteolytic enzymes (25) and therefore should also sustain the presentation of a foreign antigen to the immune system over a long time.
GFP was used in the present study as a model antigen in our system to show that RV N can serve as a carrier of a whole foreign protein. The N-GFP fusion protein was efficiently incorporated into RV RNPs and virions. As shown below, humoral responses were only detected when GFP was presented by RV RNPs, and no B cell responses were detected by recombinant GFP alone, even after three immunizations with recombinant GFP.
Materials and Methods
Plasmid Construction and Generation of Recombinant Viruses. The plasmids pBNSP, pSPBN, and pSPBN-333 have been described previously (26). To construct a recombinant RV that allows the cloning of a foreign gene in frame to the 3′ end of RV N, pBNSP was digested with PstI and NheI, and the ≈1.6-kb fragment was replaced with a ≈1.6-kb PCR-amplified product from pBNSP using Vent polymerase (Biolabs, Northbrook, IL) and the primers RP201 5′-AAAACGTACGTGAGTCACTCGAATATGTCTTG-3′ and RP64 5′-TGTGCTGCAAGGCGATTAAG-3′. The resulting plasmid was designated pBNSP-Nfu and used to introduce the GFP gene. The EGFP sequence was amplified by PCR using the primers RP199 5′-TTTCGTACGGTGAGCA AGGGCGAGGAGCTG-3′and RP200 5′-A A AGCTAGCTTACTTGTACAGCTCGTCCATG-3′ and cloned in frame with the RV N gene using the BsiWI and NheI restriction sites. The construct was named pBNSP-Nfu-GFP. The recombinant viruses were recovered as described previously (26) and designated BNSP-Nfu and BNSP-Nfu-GFP. To clone the sequence encoding the Nfu-GFP fusion protein as an additional gene between the RV G and L genes, this Nfu-GFP sequence was amplified by PCR from pBNSP-Nfu-GFP using the primers RP266 5′-TTTGGTACCACAATGGATGCCGACAAGATTG-3′ and RP200. The ≈2-kb PCR product was digested with Acc65I and NheI and cloned into pSPBN-333 digested with BsiWI and NheI. The resulting plasmid was designated pSPBN-Nfu-GFP. The recombinant virus was recovered as described previously (26) and designated SPBN-Nfu-GFP. To construct a plasmid encoding the genome of an RV expressing EGFP as an extra gene between the RV G and L gene, the EGFP sequence was amplified by PCR using the primers RP83 5′-TTTCGTACGATGGTGAGCAAGGGCGAG-3′and RP84 5′-CCCGCTAGCTTACTTGTACAGCTCGTCC-3′, digested with BsiWI and NheI, and cloned into pSPBN. The resulting plasmid was named pSPBN-GFP and recovered virus from this plasmid SPBN-GFP virus.
Sucrose Purification of RV Virions. BSR cells (a BHK cell clone) (1.25 × 107) in a T150 flask were infected for 72 h at a multiplicity of infection of 1 with SPBN-Nfu-GFP virus or SPBN; the supernatants were preclarified at 1,400 rpm for 5 min and spun over 20% sucrose in an SW27 rotor (Beckman) at 21,000 rpm for 1 h at 4°C. Virion pellets were resuspended in 200 μl of PBS.
RV RNP. BSR cells in six T150 (150 cm2) tissue culture flasks were infected with SPBN virus or SPBN-Nfu-GFP virus at an MOI of 0.01 for 72 h. The infected cells were then washed once in PBS (pH7.4), scraped from the flask and resuspended in 4 ml ice-cold distilled water containing aprotinin (50 mg/ml) (27). The cells were homogenized at 20,500 rpm, and cell debris was pelleted by centrifugation at 3,000 rpm. Supernatants were removed and saved, and the pellet was resuspended in 3.5 ml of ice-cold distilled water containing aprotinin (50 mg/ml) and homogenized again. Cell debris was again pelleted, and supernatants were combined and clarified by centrifuging in an SW-27 rotor (Beckman) for 10 min at 10,000 rpm and 4°C. The supernatant was collected, and 100 μl of 10× PBS was added before being combined with 3 g of cesium chloride and centrifuged overnight in an SW-55 rotor (Beckman) at 36,000 rpm and 19°C. The RNP bands were collected through the bottom of the tube using a 23-gauge needle. Protein concentration was determined using the BCA protein determination kit (Pierce).
Western Blotting. Virions. Proteins from lysed virions were separated in 10% SDS/PAGE gel and transferred to a poly(vinylidene difluoride)-plus membrane (Osmonics, Minnetonka, MN). Blots were blocked for 1 h in 5% dry milk powder in PBS (pH 7.4), then washed three times using Western blot wash solution [WBWS (0.1% PBS-Tween 20)] and incubated with a monoclonal mouse α-GFP antibody (1:1,000) (Sigma) or a polyclonal rabbit α-RV RNP antibody (1:2,000) overnight at 4°C. Blots were then washed three times with WBWS, and secondary goat anti-mouse IgG (1:10,000) or goat anti-rabbit IgG (1:25,000) (Jackson ImmunoResearch) conjugated to horseradish peroxidase were added. Blots were then incubated for 1 h at room temperature, and after three 10-min washes with WBWS and one wash with PBS (pH 7.4), chemiluminescence was performed as instructed by the manufacturer (Perkin–Elmer). Blots were read on X-Omat AR film (Kodak).
RNP fractions. RV RNPs were purified from BSR cells infected with SPBN or SPBN-Nfu-GFP as described above and collected in 250-μl fractions. The fractions were dialyzed against PBS two times overnight at 4°C. Proteins from purified RNPs were separated on a 10% SDS/PAGE gel and transferred to a poly(vinylidene difluoride)-plus membrane. Blots were blocked for 1 h in 5% dry milk powder in PBS (pH 7.4), then washed three times using WBWS and incubated with a monoclonal mouse α-GFP antibody (1:1,000) (Sigma) or a polyclonal rabbit RV α-RNP antibody (1:2,000) overnight at 4°C. Blots were then washed three times with WBWS. Secondary goat anti-mouse IgG (1:10,000) or goat anti-rabbit IgG (1:25,000) conjugated to horseradish peroxidase was added, and blots were incubated for 1 h at room temperature. After three 10-min washes with WBWS and one wash with PBS (pH 7.4), chemiluminescence was performed as listed previously.
One-Step Growth Curve. BSR cells were plated in 60-mm dishes and 16 h later infected at a multiplicity of infection of 5 with SPBN virus or SPBN-Nfu-GFP virus. After incubation at 37°C for 1 h, the inocula were removed, and cells were washed four times with PBS to remove any unabsorbed virus. Three milliliters of complete medium was added back, and 100 μl of tissue culture supernatants were removed at the indicated time points after infection. Virus aliquots were titered in duplicate on BSR cells.
CD4 Depletion. Six C57BL/6J female mice (The Jackson Laboratory) aged 6–8 weeks were depleted of CD4 cells before immunization by treating mice with GK1.5 (28) ascites. Mice were injected i.p. with 40 μl of GK1.5 mouse ascites diluted in 160 μl of PBS on days -3, -1, and 11 and with 12 μl of GK1.5 ascites diluted in 198 μl of PBS on days 6 and 18 postimmunization. CD4 depletion was confirmed by flow cytometry.
Immunization. Three groups of five C57BL/6J female mice aged 6–8 weeks were immunized i.m. with 5 μg of Nfu-GFP, 5 μg of wild-type RV RNP plus 2 μg of recombinant GFP (BD Biosciences), or 2 μg of recombinant GFP only. All groups of mice were boosted with the same amounts as used for initial priming, 2 and 5 weeks after primary immunization. Mice were bled retroorbitally on days indicated, and sera were analyzed by ELISA for RNP or GFP-specific antibodies as described below.
Two groups of five C57BL/6J female mice (The Jackson Laboratory) aged 6–8 weeks (one group was CD4 depleted) were immunized i.m. with 10 μg of Nfu-GFP and given a boost on day 12 with 10 μg of Nfu-GFP. All mice were bled retroorbitally as indicated and analyzed by ELISA for RNP, or GFP-specific antibodies as described below.
ELISA of Anti-RV RNP and Anti-GFP Antibody Titers. RV RNP and GFP-specific antibodies present in mice after immunization were analyzed by ELISA using 5 μg of recombinant GFP (BD Bioscience) or 10 μg of RV RNP diluted in 10 ml of coating buffer (50 mM Na2CO3, pH 9.6) as the trapping antigen. Ninety-six-well plates (Maxisorp plates, Nunc) were coated with 100 μl in each well and incubated overnight at 4°C. Plates were blocked by incubating for 30 min at room temperature using 5% milk powder in PBS followed by three washes in 0.1% PBS-Tween 20. Serum dilutions were prepared and added to wells as shown in the respective figures and incubated for 1 h at room temperature followed by three washes again in 0.1% PBS-Tween 20. Secondary antibody, goat anti-mouse IgG horseradish peroxidase (1:5,000) (Jackson ImmunoResearch) or goat antimouse IgM horseradish peroxidase (1:5,000) (Sigma) was added and incubated for 30 min at room temperature followed by three washes in 0.1% PBS-Tween. O-Phenylendiaminedihydrochloride (Sigma) was used to develop horseradish peroxidase-conjugated antibodies for 15 min and stopped by adding 2 M H2SO4. Plates were read at 490 nm using a DuPont kinetic microplate reader.
Results
Rabies Virus Ribonucleoprotein as a Carrier for Foreign Proteins. RV RNPs are structurally stable, easy to purify, and excellent B cell antigens and therefore a good source for protein production and vaccines. The finding that a segment of the C terminus of RV N (contained in RNP) is cleaved by trypsin treatment (25) suggested that this portion of RV N is probably not bound to the viral RNA and therefore might be a good site at which to add foreign peptides. The RV N C terminus was also chosen based on the analogous finding that the deletion of 18 aa from the C terminus of New Castle disease virus nucleoprotein, another negative-strand RNA virus, did not affect the production of infectious New Castle disease virus (29).
To construct a recombinant RV that allows the addition of foreign sequences fused to the RV N protein, a new RV vector was generated and used (BNSP-Nfu virus, Fig. 1). The plasmid pBNSP-Nfu was constructed in such a way that the stop codon of N was deleted and two restriction sites were introduced, which made it possible to add a foreign amino acid sequence at the C terminus of RV N. In the process of deleting the RV N stop codon, four foreign amino acids were added to the C terminus of the RV N due to the BsiWI and NheI restriction sites. However, we were able to recover BNSP-Nfu virus, indicating that there are no stringent sequence requirements for the RV N protein C terminus for RNA transcription, replication, or viral assembly. In the next step, we cloned the coding sequence of GFP as a model protein into BNSP-Nfu resulting in pBNSP-Nfu-GFP (Fig. 1). Multiple attempts to recover BNSP-Nfu-GFP virus failed, suggesting that the Nfu-GFP fusion protein may have failed to functionally replace wild-type RV N in the viral life cycle. Based on the assumption that an authentic N terminus still allows the incorporation of the recombinant protein into RV RNPs, we decided to express the Nfu-GFP protein from an extra gene, between G and L, from the RV genome (Fig. 1, SPBN-Nfu-GFP virus). We were able to recover recombinant SPBN-Nfu-GFP virus.
Fig. 1.
Recombinant RVs expressing N fusion proteins. An RV vector, which allows the expression of an RV N fusion protein, was constructed by deleting the RV N stop codon and introducing the two single restriction sites BsiWI and NheI (BNSP-Nfu). This vector site was the target for introducing the coding sequence of GFP (BNSP-Nfu-GFP). Another RV-based vaccine vector expressing an N-GFP fusion protein from an extra gene in the RV genome was constructed by introducing the gene encoding Nfu-GFP as a new RV transcription unit in pSPBN (pSPBN-Nfu-GFP), resulting in the virus vector SPBN-Nfu-GFP. The SPBN-GFP vector schematic shows an RV vector expressing GFP alone from the same site.
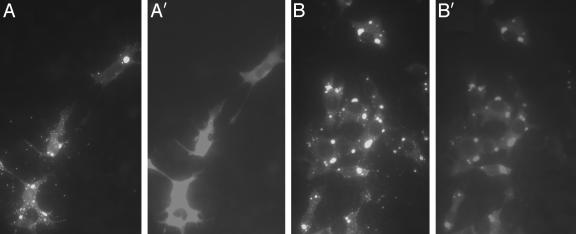
RV N-GFP Fusion Colocalizes With RV N Within Infected Cells. Initially, we attempted to analyze the recombinant Nfu-GFP protein expressed as an extra gene product by SPBN-Nfu-GFP virus in BSR cells (SPBN-Nfu-GFP virus, Fig. 1). An RV virus expressing wild-type GFP as an extra gene served as a control virus (SPBN-GFP virus, Fig. 1). As shown in Fig. 2, immunostaining with an RV anti-N-specific antibody shows RV N mainly contained in intracellular inclusion bodies, which have been proposed as sites of viral replication and transcription (Fig. 2 A and B). Likewise, a large amount of the recombinant Nfu-GFP fusion protein expressed by SPBN-Nfu-GFP virus showed a similar localization as RV N (Fig. 2B′), indicating that the RV N portion of the fusion protein is able to redirect the protein to the sites of RV replication. In contrast, in SPBN-GFP virus-infected BSR cells, immunostained specifically for GFP, resulted in the detection of a diffuse distribution of GFP within the infected cell (Fig. 2 A′).
Fig. 2.
Recombinant N-GFP fusion protein colocalizes with RV N. BSR cells were infected with virus vector SPBN-GFP (A and A′) or SPBN-Nfu-GFP (B and B′). Cells were fixed 2 days later and immunostained with a monoclonal antibody directed against GFP (A′ and B′) or an antibody specific for RV N protein (A and B). The results indicated that wild-type GFP has a diffuse distribution within the infected cell, whereas the Nfu-GFP fusion protein localizes similar to RV N protein.
The RV N Serves a Carrier to Incorporate GFP into RV RNPs. The results presented above suggested that the RV N moiety of the Nfu-GFP fusion protein is able to direct a foreign protein to the sites of RV replication. To analyze whether the recombinant RV Nfu-GFP fusion protein is incorporated into RV RNP, lysates of SPBN-Nfu-GFP or SPBN infected cells were subjected to isopycnic centrifugation, which revealed that RNPs from SPBN or SPBN-Nfu-GFP formed a sharp band at identical densities. Of note, the RNP band derived from SPBN-Nfu-GFP virus-infected cells fluoresced green during exposure to light, indicating the presence of functional GFP in the RNP complex (Fig. 3A). When fractions were collected from each CsCl gradient and analyzed by Western blotting using an RV anti-N-specific antibody, the analysis revealed that the GFP immunoreactivity completely overlapped with the RV immunoreactivity, clearly indicating incorporation of Nfu-GFP into the RV RNP complex (Fig. 3B).
Fig. 3.
Characterization of recombinant RV expressing Nfu-GFP. (A) To analyze whether the recombinant Nfu-GFP protein is associated with RV RNPs, RNPs from SPBN-Nfu-GFP infected cells were purified by CsCl density centrifugation. A sharp band, which fluoresces green during exposure to light, can be seen. (B) Twelve fractions were collected from the CsCl gradient, dialyzed, and resolved by SDS/PAGE. Probing a Western blot with an RV N-specific antibody detected the highest concentration of RNPs in fraction 9, similar to the results seen for wild-type RNPs (not shown). In addition, a GFP-specific antibody detected equal amounts of Nfu-GFP and N in all fractions, further indicating incorporation of Nfu-GFP into RV RNPs. (C) Virions from BSR cells infected with SPBN or SPBN-Nfu-GFP were purified over 20% sucrose, separated by SDS/PAGE, and analyzed by Western blotting. The results indicate that the Nfu-GFP protein is incorporated into RV virions.
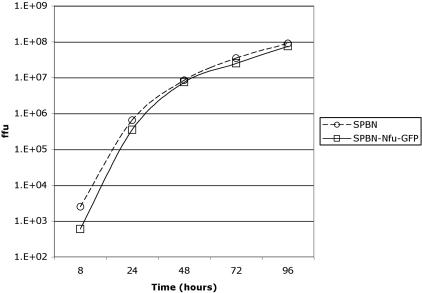
Recombinant Nfu-GFP RNPs Are Incorporated into RV Virions and Do Not Affect Viral Growth in Vitro. It was unclear whether the large amount of Nfu-GFP expressed from the extra gene in the recombinant RNPs would prevent or interfere with their packaging of RV virions. To analyze whether recombinant RNPs containing Nfu-GFP were also incorporated into RV virions, cells were infected with SPBN virus or SPBN-Nfu-GFP virus at a multiplicity of infection of 1 for 72 h, and virions were purified over 20% sucrose. Virion proteins were separated by SDS/PAGE, transferred to a poly(vinylidene difluoride)-plus membrane, and analyzed by Western blots using antibodies directed against RV N or GFP. The results in Fig. 3C show one band of 50 kDa as expected for RV N for both viruses. However, an additional band of ≈80 kDa was detected in virions from SPBN-Nfu-GFP-infected cells. Immunoblotting with a monoclonal antibody directed against GFP also detected a protein migrating at 80 kDa, confirming that the detected 80-kDa protein was Nfu-GFP. Using a Molecular Imager to measure the amount of Nfu-GFP in RNP, it was determined that Nfu-GFP was ≈30% of wild-type N in recombinant RV virions, which was similar in amount to the expression levels seen in SPBN-Nfu-GFP-infected cells. As listed above, the major concern with this approach was that the recombinant fusion protein might interfere with the viral life cycle, resulting in low or unstable virus. This result confirmed that recombinant RVs are not excluded from viral assembly but that there was still the possibility that viral titer would be affected. Because our immunostaining did not indicate any reduction in production of viral protein (Fig. 2), we decided to analyze the viral replication cycle by a one-step growth curve. Therefore, BSR cells were infected with SPBN or SPBN-Nfu-GFP at a multiplicity of infection of 5, and samples were collected at different times as indicated in Fig. 4. The results demonstrate that both viruses show approximately the same viral titer at all five time points. Therefore, neither the larger genome of SPBN-Nfu-GFP virus nor the expression of two different nucleoproteins (N and Nfu-GFP) had any major impact on viral replication or assembly. These results further confirm the high flexibility of RV genome to carry new proteins with minimal effects on their life cycle.
Fig. 4.
One-step growth curve of RV vectors. BSR cells were infected with SPBN or SPBN-Nfu-GFP at a multiplicity of infection of 5. Aliquots of culture supernatants were collected at the indicated time points postinfection, and viral titers were determined in duplicate on BSR cells.
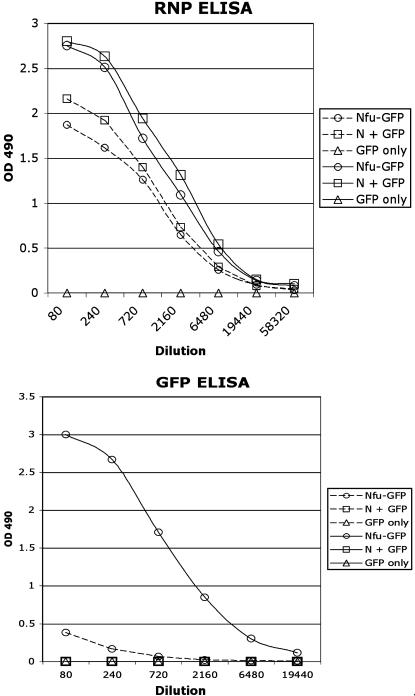
Recombinant RV RNPs Containing GFP Are Highly Immunogenic in Mice. As indicated above, RV RNPs are highly immunogenic. To study the immunogenicity of the recombinant RNPs containing GFP, groups of five 6-week-old female C57 mice were immunized i.m. with 5 μg of recombinant RNPs derived from SPBN-Nfu-GFP virus, or 2 μg of GFP alone. To examine the immunostimulating effect of wild-type RNP itself, five control mice were inoculated with a mixture of 2 μg of recombinant GFP and 5 μg of RV wild-type RNPs. Mice were boosted at weeks 2 and 5. Neither a humoral response against RV N or seroconversion against GFP was detected in any of the three groups of mice after priming. However, the first boost with the same antigens as used for priming resulted in a strong RV N-specific immune response in both groups of mice immunized with RNP (Fig. 5, RNP ELISA, dotted lines). In addition, a GFP-specific humoral response was detected in the sera of mice immunized with Nfu-GFP (Fig. 5, GFP ELISA, dotted line). The second boost, on the other hand, resulted in a dramatic increase in GFP-specific responses in the case of RNP-Nfu-GFP-immunized mice (Fig. 5, solid lines) and also enhanced the RNP-specific responses (RNP ELISA, solid lines). Again, no GFP-specific immune response was detected in mice immunized with the mixture of GFP/RNPs or GFP alone (Fig. 5, □ and ▵). These data indicated that immunization with an RNP-Nfu-protein greatly enhanced the immune responses directed to the foreign protein (GFP).
Fig. 5.
Comparison of GFP immunogenicity using different approaches. Groups of five mice were primed with RNPs containing the RV N-GFP fusion protein (Nfu-GFP, ○), a mixture of recombinant GFP protein and wild-type RV RNPs (N + GFP, □), or GFP protein only (GFP only, ▵). Because no seroconversion was detected after priming, the data points for the prime (first immunization) are not shown in this graph. The same antigens were applied in a second (1st boost, dotted lines) and third (2nd boost, solid lines) immunization. (Upper) RNP-specific ELISA. (Lower) GFP-specific ELISA.
To assess whether the observed humoral response depended on T cell help, a group of five mice was CD4+ T cell-depleted, whereas another group of nondepleted mice served as a control. All mice were immunized i.m. with 10 μg of Nfu-GFP and given boosts at day 12 with the same antigen. Mice were bled at days 0, 4, 8, 12, 16, 20, and 24 and analyzed by ELISA for RNP or GFP-specific IgM. No GFP-specific IgM was detected for any group at any time point. However, low but significant anti-RNP IgM antibodies were detected in the non-T-cell-depleted mice 4 days after the immunization boost (data not shown). These results indicated that the detected anti-RNP and anti-GFP antibody production depended on T cell help.
Discussion
Long-lasting humoral immune responses depend on both the activation of B cells and T cell helper responses. The RV N is an excellent B cell antigen, and it tightly encapsidates the RV genome into an RNase-resistant RNP core (23, 25, 30). Antigens presented in such a tightly, highly organized structure are often sufficient to activate B cells to proliferate and induce an IgM response (21, 22). This has been shown for antigens like RV G and VSV G, which are highly immunogenic if presented by the viral particle but do not activate B cells as soluble antigens (31, 32). This seems to be different for a highly organized antigen as RV RNP. We observed that GFP presented to B cells by the recombinant RNPs acts as a T cell-dependent antigen because no significant anti-GFP IgM production was detected in CD4+ T helper cell-depleted mice. In addition, we were not able to detect anti-N IgM production in these mice, suggesting that RV RNPs, in general, act as a T cell-dependent antigen. Therefore, it is likely that mechanisms other than T helper cell-independent responses are involved in the observed strong B cell response to antigens presented in such highly ordered structures as the RNP.
The insertion of foreign epitopes into viruses or virus-like particles was already shown in a variety of ways. The core antigen of hepatitis B (HBcAg) in virus-like particles has been constructed to contain a foreign epitope from hepatitis B surface antigen (HBsAg) (16), papillomavirus (18), or Plasmodium falciparum (15). Another approach uses recombinant influenza virus in which epitopes of HIV-1 gp41 (19) or Plasmodium yoelii (17) are incorporated into the hemagglutinin antigen. Additionally, a variety of foreign peptides have been chemically coupled to HBcAg and bacteriophage Qβ virus-like particles (33, 34). Even though these approaches confirmed that presentation of the foreign epitopes in such a manner induces strong B cell responses, the addition of antigens is in general restricted to relatively short peptides. In contrast, RV-based vectors cannot only incorporate whole proteins in its envelope but it can, as shown here, incorporate antigens into recombinant RNPs (12, 13, 35). Because RV-derived RNPs are easily produced in the milligram amounts, with this approach it is possible to produce large amounts of intact, whole antigens as immunogens. It is also noted that another advantage of RV RNPs might be the high stability of RNPs resulting in long-term presentation of the foreign antigen to the immune system.
Previous studies indicate that RV RNP-specific T helper cells augment the activity of virus neutralization antibody-producing B cells (23). Mice primed with RNPs followed by a boost with killed RV virions produce about a 10-fold higher B cell response compared with RNP priming followed by a boost with RV G that is found in killed RV virions (23). These results indicate the importance of the physical link of the two RV N and G antigens. We made a similar finding using recombinant RNPs, and our results indicated that RV N did not function as an adjuvant because the addition of wild-type RV RNP was unable to enhance the GFP-specific B cell response when administrated as two separate proteins.
Beside the observed strong B cell response against an antigen presented by RV RNP, this study has other important implications. Our data suggest that the C terminus of RV N seems to be quite flexible, and the addition of a whole protein to N does not affect the viral life cycle even though about one-third of the total N protein in RNP is the Nfu-GFP fusion protein. This finding adds further to the previous studies indicating that large and even multiple genes can be added to the genome of rhabdoviruses (for review see ref. 12).
This study further indicates the usefulness of RV as a vaccine vector. Our previous studies showed that RV-based vectors are able to induce potent cellular and humoral immune responses. These approaches, however, require the use of a live viral RV vaccine vehicle or killed RV virions containing a foreign glycoprotein. Because the incorporation of a foreign protein into the rhabdoviral envelope requires surface expression, this approach is only feasible for certain antigens (12). Moreover, the use of a live viral vaccine vector is only considered when killed vaccines fail to induce immunity. The ability to construct and use recombinant RVs containing RV N-fusion proteins enables us now to further analyze this approach to protect against other infections, which require strong humoral responses. Current effort of our laboratory utilizes this vaccine strategy to generate a potential Anthrax vaccine.
Acknowledgments
We thank Dr. William Wunner for critical reading of the manuscript and helpful suggestions. We also thank Dr. Robert Korngold and Christine DiRienzo for the gift of the GK1.5 monoclonal mouse antibody. This work was supported in part by National Institutes of Health Research Grants AI49153 and AI44340 (to M.J.S.) and AI45097 (to B.D.).
Abbreviations: RV, rabies virus; RNP, ribonucleoprotein; N, nucleocapsid protein; G, transmembrane glycoprotein; L, viral RNA-dependent RNA polymerase.
References
- 1.Wagner, R. R. & Rose, J. K. (1996) in Fields Virology, ed. Knipe, D. M. (Lippincott, New York), Vol. 1, pp. 1121-1136. [Google Scholar]
- 2.Conzelmann, K. K., Cox, J. H., Schneider, L. G. & Thiel, H. J. (1990) Virology 175, 485-499. [DOI] [PubMed] [Google Scholar]
- 3.Tordo, N. & Kouknetzoff, A. (1993) Onderstepoort J. Vet. Res. 60, 263-269. [PubMed] [Google Scholar]
- 4.Tordo, N., Poch, O., Ermine, A., Keith, G. & Rougeon, F. (1988) Virology 165, 565-576. [DOI] [PubMed] [Google Scholar]
- 5.Mebatsion, T., Weiland, F. & Conzelmann, K. K. (1999) J. Virol. 73, 242-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietzschold, B., Wunner, W. H., Wiktor, T. J., Lopes, A. D., Lafon, M., Smith, C. L. & Koprowski, H. (1983) Proc. Natl. Acad. Sci. USA 80, 70-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mebatsion, T., Schnell, M. J., Cox, J. H., Finke, S. & Conzelmann, K. K. (1996) Proc. Natl. Acad. Sci. USA 93, 7310-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGettigan, J. P., McKenna, P. M. & Schnell, M. J. (2002) Clin. Lab. Med. 22, 799-820. [DOI] [PubMed] [Google Scholar]
- 9.McGettigan, J. P., Foley, H. D., Belyakov, I. M., Berzofsky, J. A., Pomerantz, R. J. & Schnell, M. J. (2001) J. Virol. 75, 4430-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnell, M. J., Foley, H. D., Siler, C. A., McGettigan, J. P., Dietzschold, B. & Pomerantz, R. J. (2000) Proc. Natl. Acad. Sci. USA 97, 3544-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGettigan, J. P., Naper, K., Orenstein, J., Koser, M., McKenna, P. M. & Schnell, M. J. (2003) J. Virol. 77, 10889-10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKenna, P. M., McGettigan, J. P. & Schnell, M. J. (2003) Curr. HIV Res. 1, 229-237. [DOI] [PubMed] [Google Scholar]
- 13.Siler, C. A., McGettigan, J. P., Dietzschold, B., Herrine, S. K., Dubuisson, J., Pomerantz, R. J. & Schnell, M. J. (2002) Virology 292, 24-34. [DOI] [PubMed] [Google Scholar]
- 14.Eckhart, L., Raffelsberger, W., Ferko, B., Klima, A., Purtscher, M., Katinger, H. & Ruker, F. (1996) J. Gen. Virol. 77, 2001-2008. [DOI] [PubMed] [Google Scholar]
- 15.Schodel, F., Wirtz, R., Peterson, D., Hughes, J., Warren, R., Sadoff, J. & Milich, D. (1994) J. Exp. Med. 180, 1037-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milich, D. R., Peterson, D. L., Zheng, J., Hughes, J. L., Wirtz, R. & Schodel, F. (1995) Ann. N.Y. Acad. Sci. 754, 187-201. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues, M., Li, S., Murata, K., Rodriguez, D., Rodriguez, J. R., Bacik, I., Bennink, J. R., Yewdell, J. W., Garcia-Sastre, A., Nussenzweig, R. S., et al. (1994) J. Immunol. 153, 4636-4648. [PubMed] [Google Scholar]
- 18.Tindle, R. W., Herd, K., Londono, P., Fernando, G. J., Chatfield, S. N., Malcolm, K. & Dougan, G. (1994) Virology 200, 547-557. [DOI] [PubMed] [Google Scholar]
- 19.Muster, T., Guinea, R., Trkola, A., Purtscher, M., Klima, A., Steindl, F., Palese, P. & Katinger, H. (1994) J. Virol. 68, 4031-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niikura, M., Takamura, S., Kim, G., Kawai, S., Saijo, M., Morikawa, S., Kurane, I., Li, T. C., Takeda, N. & Yasutomi, Y. (2002) Virology 293, 273-280. [DOI] [PubMed] [Google Scholar]
- 21.Bachmann, M. F., Hengartner, H. & Zinkernagel, R. M. (1995) Eur. J. Immunol. 25, 3445-3451. [DOI] [PubMed] [Google Scholar]
- 22.Bachmann, M. F., Rohrer, U. H., Kundig, T. M., Burki, K., Hengartner, H. & Zinkernagel, R. M. (1993) Science 262, 1448-1451. [DOI] [PubMed] [Google Scholar]
- 23.Hooper, D. C., Pierard, I., Modelska, A., Otvos, L., Jr., Fu, Z. F., Koprowski, H. & Dietzschold, B. (1994) Proc. Natl. Acad. Sci. USA 91, 10908-10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dietzschold, B., Wang, H., Rupprecht, C. F., Celis, E., Tollis, M., Ertl, H. & Koprowski, H. (1987) Proc. Natl. Acad. Sci. USA 84, 9165-9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider, L. G., Dietzschold, B., Dierks, R. E., Matthaeus, W., Enzmann, P. J. & Strohmaier, K. (1973) J. Virol. 11, 748-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGettigan, J. P., Pomerantz, R. J., Siler, C., McKenna, P. M., Foley, H. D., Dietzschold, B. & Schnell, M. J. (2003) J. Virol. 77, 237-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dietzschold, B. (1996) in Laboratory Techniques in Rabies, eds. Meslin, F. X., Kaplan, M. M. & Koprowski, H. (World Health Organization, Geneva), pp. 175-180.
- 28.Dialynas, D. P., Wilde, D. B., Marrack, P., Pierres, A., Wall, K. A., Havran, W., Otten, G., Loken, M. R., Pierres, M., Kappler, J., et al. (1983) Immunol. Rev. 74, 29-56. [DOI] [PubMed] [Google Scholar]
- 29.Mebatsion, T., Koolen, M. J., de Vaan, L. T., de Haas, N., Braber, M., Romer-Oberdorfer, A., van den Elzen, P. & van der Marel, P. (2002) J. Virol. 76, 10138-10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wunner, W. H., Larson, J. K., Dietzschold, B. & Smith, C. L. (1988) Rev. Infect. Dis. 10, S771-S784. [DOI] [PubMed] [Google Scholar]
- 31.Dietzschold, B., Wiktor, T. J., Wunner, W. H. & Varrichio, A. (1983) Virology 124, 330-337. [DOI] [PubMed] [Google Scholar]
- 32.Bachmann, M. F. & Zinkernagel, R. M. (1996) Immunol. Today 17, 553-558. [DOI] [PubMed] [Google Scholar]
- 33.Jegerlehner, A., Storni, T., Lipowsky, G., Schmid, M., Pumpens, P. & Bachmann, M. F. (2002) Eur. J. Immunol. 32, 3305-3314. [DOI] [PubMed] [Google Scholar]
- 34.Fehr, T., Skrastina, D., Pumpens, P. & Zinkernagel, R. M. (1998) Proc. Natl. Acad. Sci. USA 95, 9477-9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schnell, M. J., Johnson, J. E., Buonocore, L. & Rose, J. K. (1997) Cell 90, 849-857. [DOI] [PubMed] [Google Scholar]