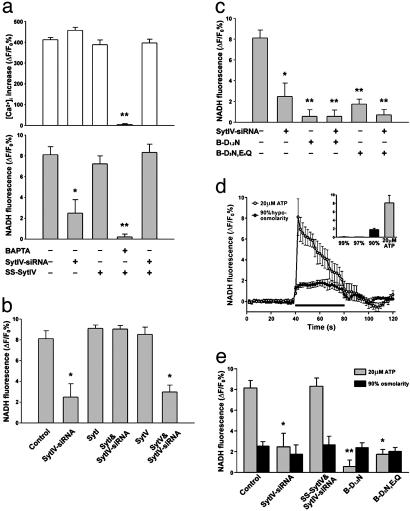
Fig. 5.
Syt IV is necessary for Ca2+-dependent glutamate release from astrocytes. (a) Expression of SS-Syt IV does not affect glutamate release or Ca2+ signaling induced by ATP (20 μM), but leads to a recovery of release when expressed in the presence of Syt IV-siRNA. Because the changes in glutamate release are independent of the amplitude of the Ca2+ signal, they support the notion that Syt IV is required downstream of the Ca2+ signal to control transmitter release (*, P < 0.05; **, P < 0.01). Data were collected from 18 (calcium imaging) and 12 (glutamate assays) trials obtained from two independent sets of cultures. (b) Syt I but not Syt V can rescue glutamate release after Syt IV-siRNA treatment (P < 0.01). (c) Mutations of amino acids homologous to Syt I C2B domain amino acids that coordinate Ca2+ binding (B-D1,2N and B-D3N,E4Q) lead to dominant-negative effects on astrocytic glutamate release (*, P < 0.05; *, P < 0.01). All modifications to release (except N,N,N′,N′-tetraacetate) are independent of changes in ATP-evoked Ca2+ mobilization. (d) Hypoosmotic saline induces the release of glutamate from astrocytes (black circles), which is comparable with ATP-evoked glutamate release (gray circles). The black horizontal bar represents the application of stimuli (ATP or hypoosmotic saline). (e) Syt IV-siRNA and Syt IV dominant-negative mutations significantly depress ATP-induced, Ca2+-dependent glutamate release (gray bar, *, P < 0.05; **, P < 0.01) but have no effect on hypoosmotic saline-induced glutamate release (black bar). Data shown in b–e were collected from 12 trials obtained from two independent cultures.