FIG. 2.
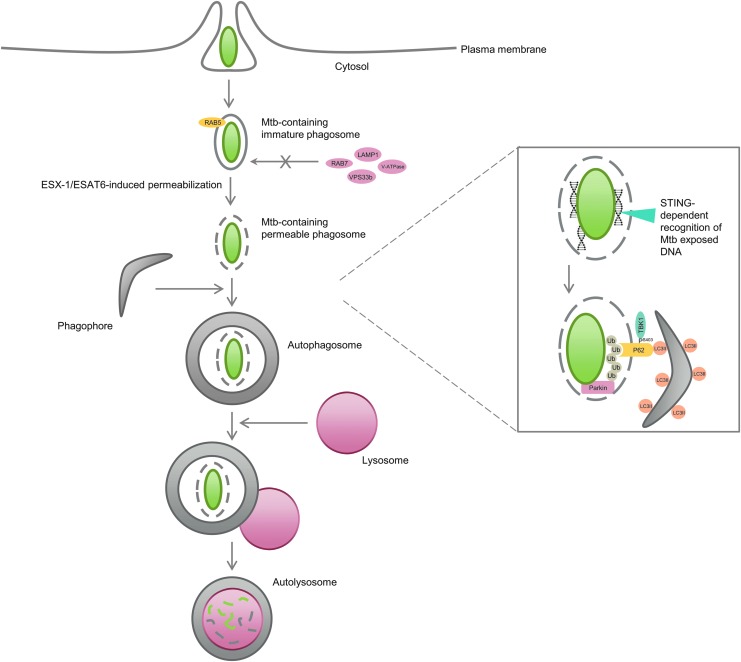
M. tuberculosis clearance by autophagy. M. tuberculosis invades macrophages by phagocytosis and arrests the maturation of the phagosome by excluding late endosome and lysosome markers (i.e., RAB7, V-ATPase, VPS33b, LAMP1) from the phagosome and by promoting the retention of early endosome markers (i.e., RAB5) in the phagocytic compartment. Host cells have developed ways of overcoming the evasion of M. tuberculosis from the phagocytic pathway by taking advantage of some intrinsic M. tuberculosis mechanisms. For instance, phagosomal permeabilization induced by the bacterial ESX-1/ESAT-6 system allows the host protein STING to recognize extracellular bacterial DNA, which then promotes ubiquitin marking of bacteria (mostly through K63-linkage chain formation by the E3 ligase Parkin). Ubiquitin is then recognized by autophagy adaptors, such as P62, which deliver the bacilli to autophagosomes. TBK-1-induced phosphorylation of Ser403 of P62 increases the affinity of P62 to ubiquitin. Autophagosomes are subsequently fused to lysosomes, where degradation of mycobacteria occurs. LAMP1, lysosomal-associated membrane protein 1; STING, stimulator of the interferon gene; TBK-1, TANK-binding kinase 1.