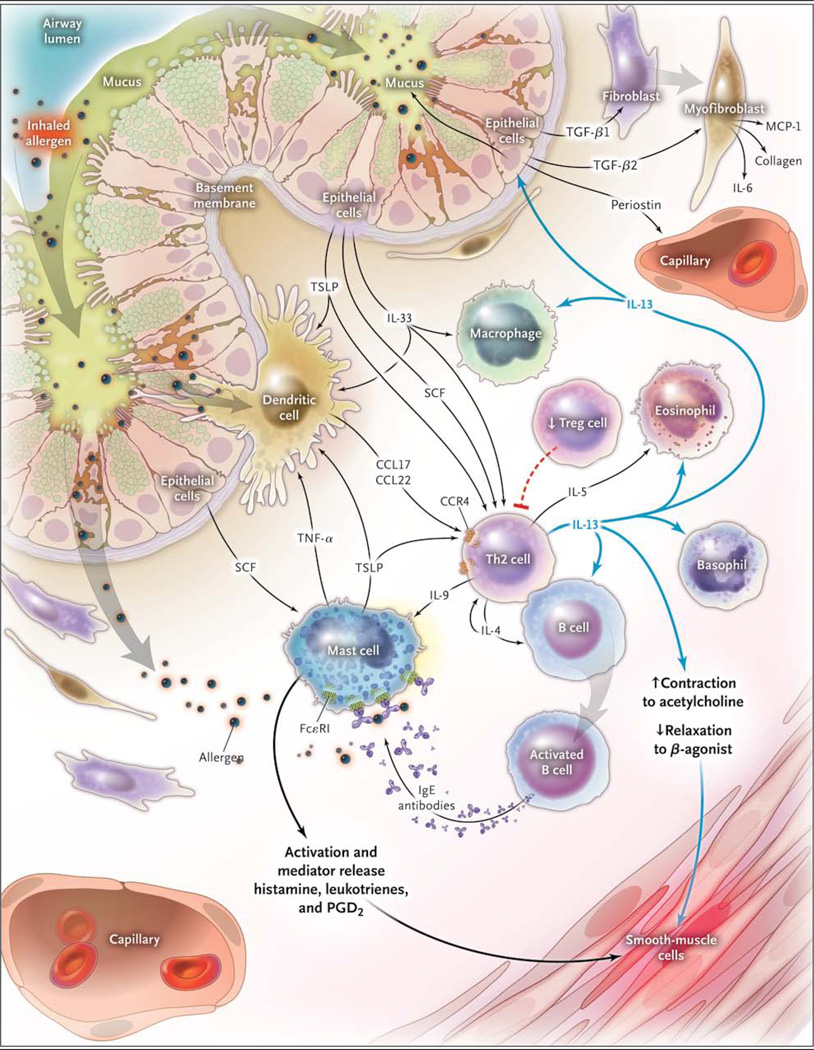
Figure 1. Interleukin-13 and Non–Interleukin-13 Inflammatory Pathways in Asthma.
Inhaled allergen activates mast cells, which are maintained by stem-cell factor (SCF) produced by epithelial cells and by dendritic cells, through cross-linking with IgE on their cell surfaces via FcεR1 to release mediators that induce bronchoconstriction, such as histamine, cysteinyl leukotrienes, and prostaglandin D2 (PGD2). Allergens are processed by dendritic cells, which are induced to secrete the CC chemokine ligand (CCL) 17 and CCL22 by thymic stromal lymphopoietin (TSLP). Dendritic cells then attract and activate type 2 helper T-cells (Th2) by the binding of CCL17 and CCL22 with CC chemokine receptor 4 (CCR4) on the Th2 cell surface. Another driver of the allergen sensitization process is interleukin-33 (IL-33), produced by airway epithelial cells, which activates dendritic cells and Th2 through mast-cell–derived tumor necrosis factor alpha (TNF-α). Th2 secrete IL-4 and IL-13, which induce B cells to produce IgE; IL-5, which is necessary for the development and survival of eosinophils; and IL-9, which activates mast cells. T regulatory (Treg) cells inhibit this inflammatory cascade, and there are data that suggest that they may be reduced in asthma, thus promoting ongoing Th2 inflammation. Once IL-13 is produced, it can promote the survival and migration of eosinophils and promotes activation of macrophages to create an M2, or an allergic cell phenotype. Through modulation of the barrier function of airway epithelial cells and subsequent production of transforming growth factor β1 (TGF-β1), the permeability of airway epithelial cells and the production of mucous are increased, and airway fibroblasts transform to myofibroblasts, with subsequent production of monocyte chemoattractant protein 1 (MCP-1), IL-6, and collagen. IL-13 also has direct effects on airway smooth muscle, leading to increased contraction to agonists such as acetylcholine and decreased relaxation with beta-agonists.