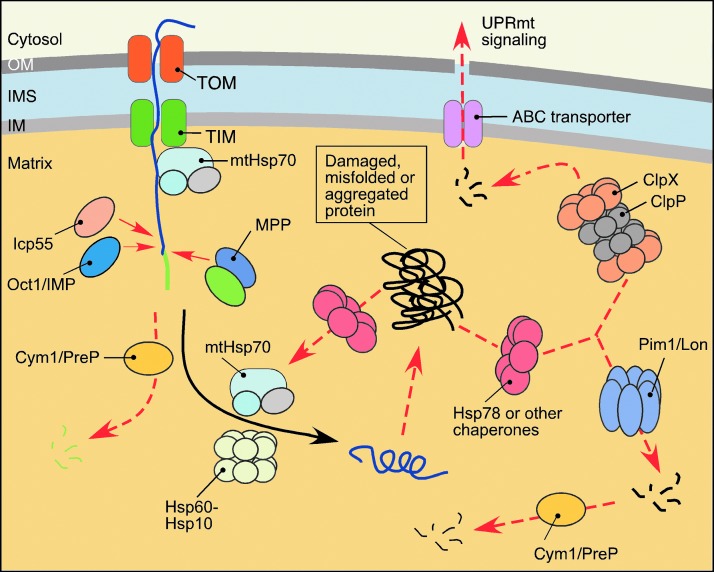
FIG. 4.
PMQC in the matrix. Multiple proteases and molecular chaperones regulate the matrix subproteome. The regulation involves control of protein maturation and accumulation and degradation of poly- and oligopeptides. Proper maturation of the precursor proteins transported via the TIM23 translocase complex requires removal of mitochondrial targeting sequence by MPP processing metallopeptidase complex and, in certain cases, additional stabilizing processing by intermediate peptidases MIP/Oct1 and Icp55. Resulting free targeting peptides, as well as other small oligopeptides, are removed by mitochondrial presequence peptidase Cym1/PreP. Subsequent protein folding is facilitated by Hsp family chaperones. Stress-damaged, misfolded, and/or aggregated proteins are recognized and cleaved by AAA+ proteases Lon/Pim1 and ClpXP. Peptides produced by these proteolytic events are either subjected to additional processing by oligopeptidases or extruded through ATP-binding cassette (ABC)-type transporters into the cytosol where they activate mitochondrial unfolded protein response (UPRmt). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars