Fig. 10.
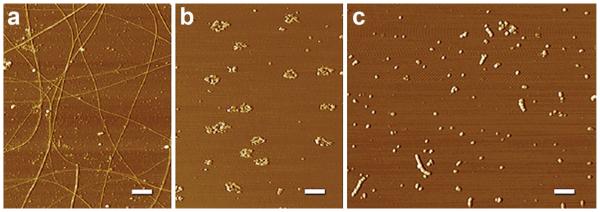
Diverse structural outcomes from different aggregation protocols. AFM images revealing a spectrum of Aβ structures are shown. a Fibrils: Aβ1–42 aged in ddH2O for one week contains periodic and smooth fibrils throughout the field of diverse sizes and lengths. b Rings: Aβ1–42 aged in F12 at 37 °C for 24 h and not centrifuged contains many ring-like structures and a few short linear protofibrils. c AβOs/protofibrils: Aβ1–42 aged in PBS for 48 h at RT contains many linear protofibrillar structures and small, spherical structures. Protofibrillar structures appear to contain bead-by-bead assemblies of multiple globular structures. The results suggest that temperature and chemical environment are critical during Aβ aggregation. Scale bar 400 nm. From Chromy et al. [16]
