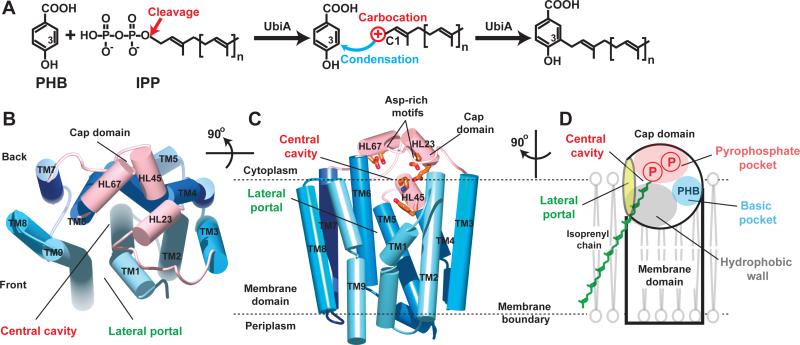
Figure 1. Scheme of UbiA catalysis and structure of ApUbiA.
(A) UbiA catalysis. Cleavage of the pyrophosphate group from the IPP substrate generates a carbocation intermediate, which reacts at the meta-position of the PHB substrate to complete the condensation reaction. (B) Apo-structure of ApUbiA viewed from the cytoplasmic side. The cap domain is shown in pink and the TMs are shown in different blue colors. (C) A side view of the same structure. Conserved residues in the central cavity are shown in orange. (D) Cartoon representation of the structure, which has a unique lateral portal that opens to membrane. The large central cavity contains a polar pocket (pink) for pyrophosphate binding, a hydrophobic wall (grey) for the binding of isoprenyl chains, and a small basic pocket (blue) for PHB binding (see fig. S5).