Abstract
Introduction
Erectile dysfunction is a major complication of radical prostatectomy, commonly associated with penile neuropathy. In animal models of peripheral nerve injury, glial growth factor-2 (GGF2), a member of the neuregulin family of growth factors, has neuroprotective and neurorestorative properties, but this potential has not been established after cavernous nerve (CN) injury.
Aims
The effectiveness of GGF2 in preserving axonal integrity and recovering erectile function in a rat model of radical prostatectomy-associated CN injury.
Methods
Adult male Sprague-Dawley rats underwent bilateral CN crush injury (BCNI) or sham surgery. Rats were administered GGF2 (0.5, 5, or 15 mg/kg) or vehicle subcutaneously 24h pre- and 24h post-BCNI, and once weekly for 5 weeks. Erectile function was assessed in response to electrical stimulation of the CN. CN survival was assessed by fluorogold retrograde axonal tracing in major pelvic ganglia (MPG). Unmyelinated axons in the CNs were quantitated by electron microscopy.
Main Outcome Measures
Erectile function recovery, CN survival, and unmyelinated CN axon preservation in response to GGF2 treatment following BCNI.
Results
Erectile function was decreased (P<0.05) after BCNI, and it was improved (P<0.05) by all doses of GGF2. The number of fluorogold-labeled cells in the MPG was reduced (P<0.05) by BCNI, and was increased (P<0.05) by GGF2 (0.5 and 5 mg/kg). The percentage of denervated Schwann cells in the BCNI group was higher (P<0.05) than that in the sham-treated group, and was decreased (P<0.05) in the GGF2 treated (5 mg/kg) BCNI group. In the BCNI+GGF2 (5mg/kg) group, the unmyelinated fiber histogram demonstrated a rightward shift, indicating an increased number of unmyelinated axons per Schwann cell compared to the BCNI group.
Conclusions
GGF2 promotes erectile function recovery following CN injury in conjunction with preserving unmyelinated CN fibers. Our findings suggest the clinical opportunity to develop GGF2 as a neuroprotective therapy for radical prostatectomy.
INTRODUCTION
Pelvic surgeries for prostate, bladder, and colorectal malignancies commonly result in a high incidence of erectile dysfunction due to trauma of the cavernous nerves (CN), the principal autonomic innervation of the penis.1 Despite surgical advances, such as nerve sparing techniques and robotic procedures, less than 40% of patients regain erectile function sufficient for sexual intercourse following bilateral CN sparing surgery.2,3
The CN originate from the major pelvic ganglia (MPG) bilaterally and contain parasympathetic and sympathetic fibers. The proximal part of the CN exiting from the MPG contains both myelinated and unmyelinated fibers, but the CN gradually contains fewer myelinated fibers at the distal level.4 At the point of crural entry, the nerve is almost exclusively composed of unmyelinated axons.4 Unmyelinated axons are, thus, part of CN fibers that provide the neurotransmitters for penile innervation, the most important one being nitric oxide (NO).5
Following radical prostatectomy, CN damage may be a consequence of mechanically induced nerve division or stretching, thermal damage, ischemia, or local inflammatory effects. Various local and systemic neuromodulatory agents have been investigated in animals modeling radical prostatectomy to protect or regenerate CN and facilitate erection recovery with findings suggesting benefits. They include immunophilin ligands, neurotrophins, growth factors, Schwann cell seeded guidance tubes, glial cell line-derived neurotrophic factor, sonic hedgehog protein, atypical neurotrophic factors, nerve guides, tissue engineering/stem cell therapy, and gene therapy.6–10 However, there are no treatments established for either CN neuroprotection or nerve regeneration at the clinical level.9 Thus, investigative efforts are ongoing to identify and develop an ideal strategy that effectively preserves CN function in this clinical context.
Neuregulins are a family of growth factors related to epidermal growth factor and function by binding ErbB tyrosine kinase transmembrane receptors (ErbB2-4).11, 12 Neuregulins stimulate cellular proliferation, differentiation, and survival in many tissues.13–16 Neuregulin-1 plays a crucial role in axoglial signaling during the development of the peripheral nervous system.11 Furthermore, this growth factor is increasingly being recognized for its neuroprotective and neurorestorative properties during adulthood, conceivably through mediating signals between axons and Schwann cells required for effective nerve repair.11, 17
Given these neuroprotective effects, neuregulins represent an attractive candidate for protecting the CN and preserving erectile function after radical prostatectomy. The objective of our study was to evaluate whether glial growth factor 2 (GGF2, neuregulin-1β3 type II), a soluble full-length splice variant of the neuregulin-1 gene,11 promotes axonal integrity and preserves erectile function following bilateral CN crush injury (BCNI) in a rat model of radical prostatectomy-associated CN injury.
MATERIALS AND METHODS
Study Design
Two experimental protocols were performed in 2 independent laboratories. The protocols employed different severities of CN crush injury and GGF2 dosing regimens, with common measurements of physiologic response and complementary techniques for evaluating neuroprotective effects of GGF2 (Table 1).
Table 1.
Experimental design in Protocols 1 and 2.
| Protocol 1 | Protocol 2 | |
|---|---|---|
| BCNI | Severe | Moderate |
| GGF2 (mg/kg) | 0; 0.5; 5 | 0; 5; 15 |
| Measurements | Erectile function | Erectile function |
| Retrograde neuronal tracing | CN unmyelinated axons |
Animals
Adult male Sprague–Dawley rats (325–350 g; Charles River Breeding Laboratories, Wilmington, MA, USA, and Montreal, Quebec, Canada) were used. All experiments were approved by the Institutional Animal Care and Use Committees.
Bilateral CN Crush Injury (BCNI) and Treatments
To perform BCNI on anesthetized rats, prostate and bilateral MPG were identified via a midline lower abdominal incision and left and right CNs were isolated. Both CNs were crushed 1–2 mm distal to the MPG. To limit variability, all surgeries were completed by the same trained investigator for each respective protocol. In Protocol 1, BCNI was induced by crushing with a serrated hemostat at a constant ‘one-click’ pressure for 2 minutes per side.18 Animals were randomly divided into 3 groups (n = 8–10/group): BCNI+vehicle; BCNI+GGF2 (0.5 mg/kg); BCNI+GGF2 (5 mg/kg). A fourth group was sham treated with vehicle (n=10). In Protocol 2, BCNI was induced by crushing with a fine-grade hemostat for 3 minutes.19 Animals were randomly divided into 3 groups (n = 10–12/group): BCNI+vehicle; BCNI+GGF2 (5 mg/kg); BCNI+GGF2 (15 mg/kg). Fourth and fifth groups were sham+vehicle and sham+GGF2 (15 mg/kg; n=10–12 each). Sham surgeries were completed by exposing the CNs but not manipulating them. GGF2 and vehicle were administered subcutaneously 24 h prior to BCNI, 24 h post BCNI, and then once weekly until study end 5 weeks post BCNI. Treatments were performed in a blinded fashion. The timing and doses of GGF2 used in these experiments were within the range used in previous studies.20, 21 Vehicle contained 20 mM sodium acetate, 100 mM sodium sulfate, 1% mannitol, and 100 mM L-arginine (pH 6.5). Recombinant human GGF2 and vehicle were provided by Acorda Therapeutics, Inc. (Ardsley, NY, USA). Erectile function measures and harvest of MPG and penis were performed 5 days after the last dose.
In Vivo Erection Studies
Intracavernosal pressure (ICP) responses induced by CN electrical stimulation were measured in a blinded fashion at 5 weeks post injury as described previously.19, 22, 23 Briefly, under anesthesia, CNs were isolated via a midline abdominal incision and the crura of the penis identified. A bipolar stainless steel electrode was placed around the CN at 1–2 mm distal to the crush site. ICP was monitored and recorded via a cannula inserted into the crura. In Protocol 1, maximum stimulation intensity was used, while in Protocol 2 increasing stimulation intensities were used. In Protocol 1, arterial blood pressure (ABP) was measured at the end of the procedure using a butterfly needle inserted into the aorta. In Protocol 2, mean arterial pressure (MAP) was continuously monitored after the cannulation of the right carotid artery with polyethylene-50 tubing. Response parameters were recorded using LabVIEW 4.0 software (National Instruments, Austin, TX, USA) or data acquisition (DI-190; Dataq Instruments, Akron, OH, USA) and calculated using MATLAB software (Mathworks, Natick, MA, USA). Maximum ICP (maximum pressure that is reached during CN electrical stimulation) and total ICP (ICP area under the curve, indicating the ICP response for the duration of CN electrical stimulation) were expressed per ABP or MAP.
Retrograde Fluorogold Tracing
Retrograde neuronal tracing with fluorogold was performed to determine the number of intact neurons in sham-treated animals and animals which underwent BCNI and were treated with GGF2 (0.5 mg/kg and 5 mg/kg) or vehicle (Protocol 1). Flurogold injected into a target organ is retrogradely transported from the nerve terminals to the nerve cell bodies if the nerve remains intact.24 Animals were anesthetized and 4 μl of 4% fluorogold (Biotium, Hayward, CA, USA) was injected into the corpora cavernosa bilaterally 7 days before the study endpoint.24 After ICP measurement, both MPG were harvested and fixed in 4% paraformaldehyde, 0.1M phosphate buffer overnight and then placed in 20% sucrose. Serial transverse sections were cut at 20 μm thickness. A subset of ten slides per animals were randomly selected and images were taken using the Infinity camera and imaging system, followed by blinded counts of fluorogold enhanced cells.
Electron Microscopy of the CN
Electron microscopic analysis was performed to assess axonal integrity of the CN in sham-treated animals and animals that underwent CN crush and were treated with GGF2 at 5 mg/kg or vehicle (Protocol 2). Anesthetized animals were perfused with 4% paraformaldehyde. Right and left dorsolateral prostate tissue along with the MPG and associated CN were removed en bloc. Tissues were postfixed with 4% paraformaldehyde-3% glutaraldehyde (Electron Microscopy Sciences, Ft Washington, PA, USA) at 4°C for 3 days. CNs distal to the crush site were dissected out and placed in phosphate buffer until processed for electron microscopy by using standard methods as described previously.25, 26 Quantification of degenerating axons was conducted as described25, 27 by a person blind to the treatment protocol. Both number of Schwann cells per CN and number of unmyelinated axons per Schwann cell were counted. The number of nonmyelinating Schwann cells was determined by the number of units of multiple small axons ensheathed by a single Schwann cell. For analysis, photographs of the entire section of one nerve from each animal were taken at a magnification of 10,000x. Total counts of unmyelinated axons as well as Schwann cell nuclei were performed on the whole cross section of the nerve.
Statistical Analysis
The data were expressed as means ± standard error of the mean (SEM). Statistical analyses were performed using one-way analysis of variances (ANOVA; SigmaStat Windows Version 3.00; Systat Software, San Jose, CA, USA), followed by Holm-Sidak multiple comparison test. A value of P< 0.05 was considered to be statistically significant.
RESULTS
GGF2 Treatment Preserves Erectile Function Following CN Injury
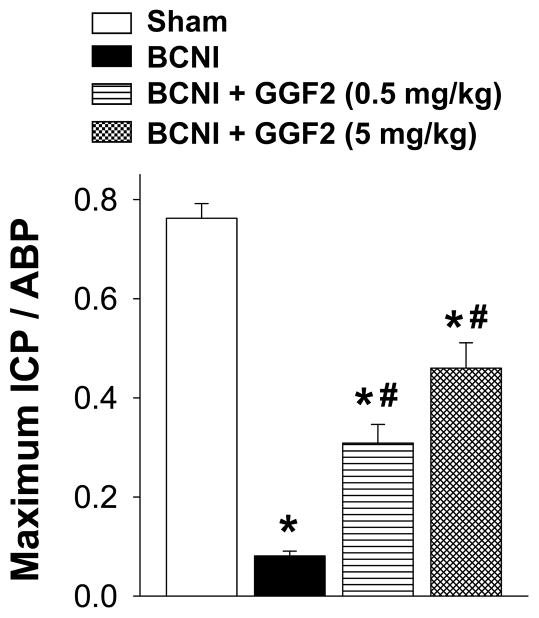
BCNI in Protocol 1 resulted in an approximately 90% decreased erectile function at 5 weeks post injury (measured as maximal ICP/ABP) compared to sham treatment (P<0.05; Figure 1), consistent with severe CN injury. GGF2 at 0.5 mg/kg and 5 mg/kg significantly (P<0.05) increased erectile function in BCNI animals, returning maximal ICP to approximately 50% of the uninjured level at 5 weeks. This group was still statistically different from animals in the sham group (P<0.05 vs sham). These results demonstrate that GGF2 improves erectile function following severe BCNI.
Figure 1.
Effect of severe bilateral CN crush injury (BCNI) and GGF2 treatment on penile erection 5 weeks after surgery. The stimulus parameters were 1.5 mA, 20 Hz (maximal stimulation intensity), pulse width 0.2 msec, and duration 50 sec. Erectile response to electrical stimulation of the CN is indicated by maximal ICP/arterial blood pressure (ABP). Stimulated and baseline ICPs (cmH20) for all treatments groups were: 136.88 ± 7.8 vs 9.12 ± 0.7 (P<0.05) in sham group; 15.13 ± 1.7 vs 7.75 ± 0.7 (P<0.05) in BCNI group; 55.25 ± 7.8 vs 6.75 ± 0.4 (P<0.05) in GGF2 (0.5 mg/kg) group, and 84.75 ± 11.4 vs 7.50 ± 0.6 (P<0.05) in GGF2 (5 mg/kg) group. Each bar represents the mean ± SEM of 7–8 rats. *P<0.05 vs Sham, #P<0.05 vs BCNI.
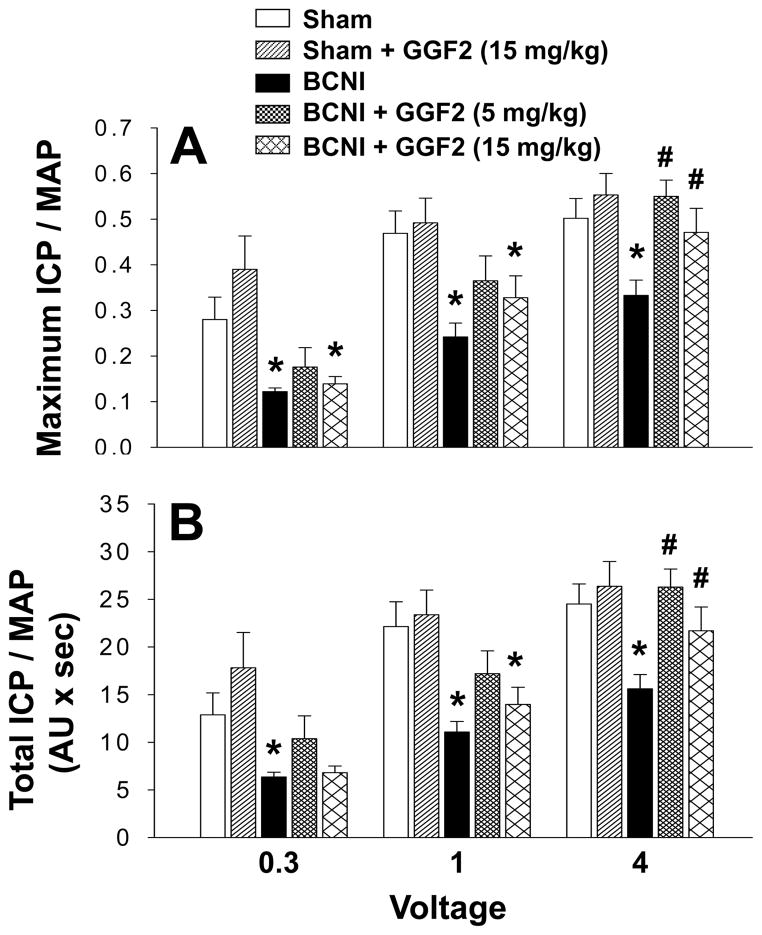
BCNI in Protocol 2 also resulted in significantly (P<0.05) decreased erectile function at 5 weeks (measured as maximal ICP/MAP and total ICP/MAP) compared to sham treatment, but only by about 40–50% (Figure 2), consistent with moderate CN injury. At low stimulation intensities (0.3 volts and 1 volt), erectile function was not significantly decreased in crush-injured rats treated with 5 mg/kg GGF2, but was significantly (P<0.05) decreased in crush-injured rats treated with 15 mg/kg GGF2 compared to that of sham treated rats. At a higher stimulation intensity (4 volts), GGF2 at both 5 mg/kg and 15 mg/kg significantly (P<0.05) increased erectile function relative to that of untreated crush-injured rats, comparable to levels of sham treated animals. GGF2 (15 mg/kg) did not produce a significant change in erectile response in sham treated rats. These results indicate that GGF2 preserves erectile function following moderate BCNI.
Figure 2.
Effect of moderate bilateral CN crush injury (BCNI) and GGF2 treatment on penile erection 5 weeks after surgery. Electrical stimulation of the CN was performed as voltage response (0.3, 1.0 and 4.0 volts) at 16 Hz with square-wave duration of 5 milliseconds for 1 minute. Erectile response to electrical stimulation of the CN is indicated by maximal ICP/mean arterial pressure (MAP, A) and total ICP/MAP (B). Each bar represents the mean ± SEM of 10–12 rats. *P<0.05 vs Sham, #P<0.05 vs BCNI. AU = arbitrary units.
GGF2 Treatment Preserves Fluorogold-positive Cells in the MPG Following CN Injury
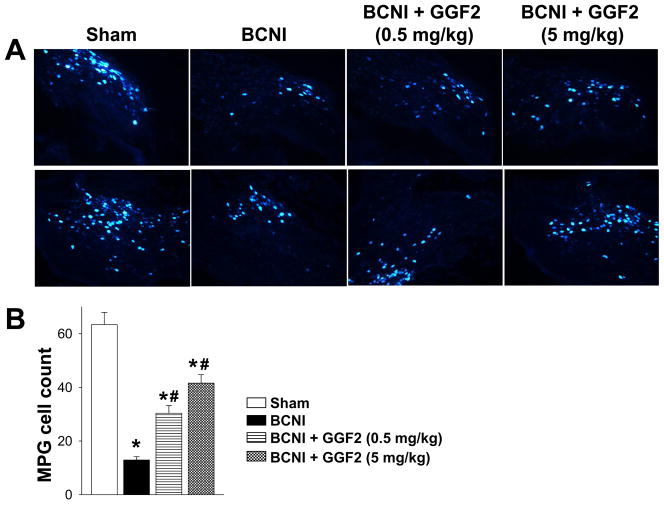
Intact neurons within the CN were assessed by retrograde fluorogold labeling (Figure 3A). In the MPG of sham treated rats, 63.4 ± 5 neurons per section were labelled (Figure 3B), as similarly reported in other studies.28, 29 Following severe BCNI and vehicle treatment (Protocol 1), the number of fluorogold-labeled cells in the MPG was reduced by almost 80% (P<0.05). Cell counts were significantly (P<0.05) increased after treatment with both 0.5 mg/kg and 5 mg/kg GGF2 by approximately 50% and 70%, respectively, though they were still significantly decreased (P<0.05) compared to the sham treated group. The results indicate that GGF2 protects nerve fibers after BCNI.
Figure 3.
Retrograde tracing using fluorogold following severe bilateral CN crush injury (BCNI) and GGF2 treatment. (A) Representative fluorogold labeling of MPG. (B) Quantification of fluorogold labeling in the MPG from 10 randomly selected slides by a blinded observer. Each bar represents the mean ± SEM of 7–8 rats. *P<0.05 vs Sham, #P<0.05 vs BCNI.
GGF2 Treatment Preserves Unmyelinated Axons Following CN Injury
Unmyelinated fiber profiles of the CNs were evaluated by counting the number of unmyelinated axons per Schwann cell per CN. In contrast to the one-to-one relationship between Schwann cells and myelinated axons, multiple unmyelinated axons can be ensheathed by a single Schwann cell.30 The average total Schwann cell count per CN was not different between sham treated (127.7 ± 23.4; n=4), CN crush-injured (112.7 ± 13.8; n=5), and GGF2 (5 mg/kg)-treated CN crush-injured (115.2 ± 19.8; n=5) groups. Thus, similar numbers of Schwann cells were analyzed per CN in each group and any difference in the number of unmyelinated fiber count was not due to differences in the number of Schwann cells included in the analysis.
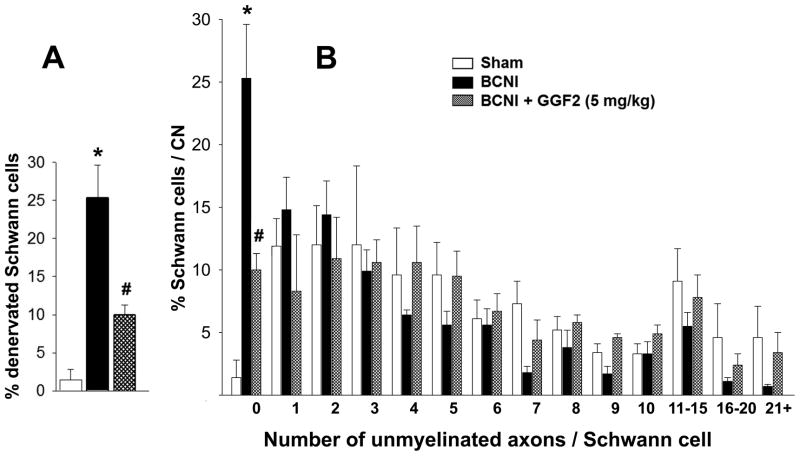
Figure 4 demonstrates percentage of Schwann cells associated with a given number of unmyelinated axons. Absence of unmyelinated axons (i.e. number of unmyelinated axon 0) indicates complete denervation of the Schwann cell. Following moderate CN crush injury (Protocol 2), the percentage of denervated Schwann cells was significantly (P<0.05) higher (25.3 ± 4.3%) than that in the sham treated group (1.4 ± 1.4%). GGF2 treatment (5 mg/kg) significantly (P<0.05) lowered the percentage of denervated Schwann cells after CN crush injury (10.0 ± 1.3%) compared to that of vehicle treatment (Figure 4A). The rightward shift toward a higher number of unmyelinated fibers/Schwann cell in the GGF2-treated CN crush-injured group histogram indicates a preservation of unmyelinated fibers in this group (Figure 4B), as previously shown in other studies.30 Representative electron microscopic images of CNs (Figure 5) show more denervated Schwann cells and more degenerating unmyelinated axons in the CN crush-injured group compared to sham-treated and GGF2-treated CN crush-injured groups. These results indicate that GGF2 preserves unmyelinated nerve fibers in the CN.
Figure 4.
Histogram showing percentage of denervated Schwann cells (A) and the distribution of unmyelinated nerve fiber numbers associated with Schwann cells (B) in the CN distal to the crush site 5 weeks after moderate bilateral CN crush injury (BCNI) and GGF2 treatment. n=4–5. *P<0.05 vs Sham, #P<0.05 vs BCNI.
Figure 5.
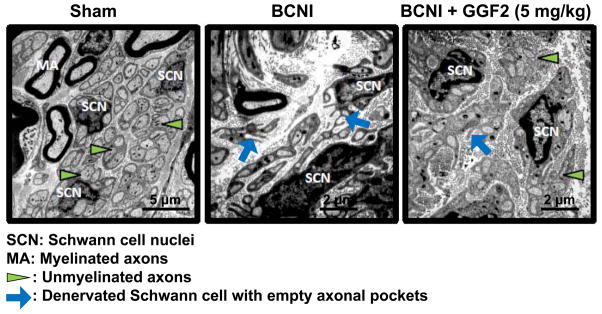
Representative electron microscopy images of CN after moderate bilateral CN crush injury (BCNI) and GGF2 treatment.
We did not measure the number of myelinated axons, observing that they represent less than 5% of the axons.
Changes in Body Weight
All rats gradually gained weight from about 325 g to about 490 g from start to end of the experiment. Sham treated and CN crush-injured animals administered GGF2 gained weight slightly more slowly than untreated animals starting at week 3 after the treatment (5th dose of GGF2), showing about a 3% reduction (P<0.05) in total weight gain. All animals appeared vigorous and healthy throughout the study. While the reason for the observed minimal weight loss is not known, GGF2 toxicology and efficacy studies have shown evidence of inappetence and emesis in several species,31 with weight gain return to a normal rate after treatment is stopped.
DISCUSSION
This study demonstrates that treatment with GGF2 starting just before CN injury and continuing with weekly dosing attenuates the deleterious impact of CN injury on erectile function at 5 weeks post injury and promotes axonal integrity in the damaged CN. This effect of GGF2 on penile innervation suggests that GGF2 may represent a new therapeutic approach to preserve or restore erectile function following radical prostatectomy.
In our study CN crush performed by 2 different investigators at independent laboratories established severe and moderate injuries. While differences in tools used to induce crush may explain differences in injury severity, it is also plausible that investigator variability played a role. Severe crush injury of the CN resulted in almost complete loss of erectile function. Despite the severity of the injury, GGF2 treatment was associated with a dose-dependent and significant improvement in erectile function returning it to approximately 50% of that of sham treated animals. Increased function of this magnitude indicates a substantial neuroprotective effect.
The moderate injury was designed to mimic partial nerve damage that occurs during nerve-sparing radical prostatectomy conceivably via mechanical stretch.32–34 Moderate CN injury resulted in about 40–50% decrease in erectile function. Impaired erectile function in this animal model is likely due to degeneration of a significant number of unmyelinated axons in injured CN and Schwann cell denervation. GGF2 treatment resulted in a significant improvement in erectile function in both the 5 mg/kg and 15 mg/kg groups.
Schwann cells are the main glial cells of the peripheral nervous system, essential to the survival and function of neurons. They are involved in the conduction of nervous impulses along axons, nerve development, trophic support for neurons, and nerve regeneration.35 Prolonged periods of time in which Schwann cells do not contact axons largely account for very poor functional recovery after peripheral nerve injuries, as denervated Schwann cells die by apoptosis or atrophy and do not support axon growth.36 In our study, GGF2 increased the number of unmyelinated axons per Schwann cell in the crushed CN and prevented degeneration of unmyelinated axons. Thus, GGF2 restored erectile function presumably either by protecting the nerve from the initial mechanical insult or attenuating the effects of subsequent pathological processes. Immunohistochemical identification of nNOS positive nerve fibers and nerve markers vesicular acetylcholine transporter and tyrosine hydroxylase in the penile corpora cavernosa (preliminary observation, data not shown) further substantiated preservation of the CN and general preservation of penile neurotransmission by GGF2.
The neuroprotective and neurorestorative effects of GGF2 have been described in several animal models of both central and peripheral nervous system injury and are thus consistent with our results. In the peripheral nervous system, GGF2 given to rats every 24 h for 4 days beginning 24 h before sciatic nerve injury, induced nerve regeneration and remyelination of axons and improved functional recovery on days 11–21 post-injury.20 In a rabbit model of facial nerve transection, treatment with GGF2 at the time of injury and 1 and 2 days post-injury, increased the number of Schwann cell nuclei, myelination, and nerve regeneration.37 In a rat model of cisplatin-induced neuropathy, characterized by a decrease in the sensory nerve conduction velocity, treatment with rhGGF2 for 11 weeks provided full neuroprotection.21
The exact targets for GGF2 and mechanisms of its action in preserving CN and erectile function following CN injury warrant further investigations. In the context of peripheral nerve injury, in vitro studies suggest the role of neuregulin-1 type II in the migration of Schwann cells and macrophages, implying a potential role of neuregulin-1 in the initial stage of Wallerian degeneration.38 GGF2-induced Schwann cell migration after nerve crush has been attributed to induction of α5β1 integrin-ErbB2 receptor-FAK complex formation.39 In addition to nerve cells,13 multiple other cells, such as endothelial, 14 smooth muscle,15 epithelial, 40 and cardiac myocytes16 express ErbB receptors and respond to neuregulins with growth and differentiation. Thus, it is possible that GGF2 may act on different cell types in the CN and in the penis to exert its neuroprotective or neuroregenerative effects.
We acknowledge that several additional research areas require investigation. The integrity of the corpus cavernosum following GGF2 treatment could be supported by quantitating nNOS content and smooth muscle/collagen ratio in the corpus cavernosum. Furthermore, localization of the ErB receptors in the MPG and corpora cavernosa would suggest the possible site of GGF2 action in protecting penile innervation. In addition, we acknowledge a limitation in our study: the integrity of the CN following GGF2 treatment could be further supported by quantitating nNOS content in the MPG.
For this study we performed pre-injury treatment to provide the best chance for acute neuroprotection, and continued with weekly dosing to maintain exposure. Further preclinical studies are needed to explore dosing regimens and routes of administration of GGF2 to optimize CN functional recovery. The dosing regimen employed in these initial studies is clinically informative suggesting the benefits of acute immediate treatment and its continuation for a substantial duration after injury. Ongoing studies will determine if the benefit represents a frank neuroprotective strategy derived mainly from acute treatment, or if the regenerative or restorative effects of GGF2 are at play in this peripheral injury setting, requiring longer term dosing after injury. It is also of clinical importance to determine the effectiveness of GGF2 given with some delay post CN injury on functional outcomes to characterize possible neurorestorative mechanisms in this model. Further studies may also serve to establish non-mitogenic effects, which have importance in oncologic situations. Nonetheless, our study displays important strengths. We employed 2 different protocols performed by independent investigators, adding robustness for the demonstrated neuroprotective effects of GGF2. We also used 3 different doses of GGF2, all showing consistent beneficial effects that were dose-related.
CONCLUSION
Treatment with GGF2 starting just before CN injury promotes the recovery of erectile function in a rat model of radical prostatectomy-associated CN injury. This occurs in conjunction with preserved unmyelinated nerve fibers in the injured CN. Our findings suggest that clinical investigation of GGF2 as a neuroprotective therapy for pelvic surgeries, including radical prostatectomy, is warranted. The ability to deliver GGF2 remotely from the surgical field and the ability to pre-treat are key advantages for potential translation to the clinical arena.
Acknowledgments
This work was supported by Acorda Therapeutics (to ALB and AJB) and NIH/NIDDK grant DK067223 (to ALB).
Footnotes
Conflict of Interest: “None” or details of Conflict
Drs Iaci and Caggiano are employees and stockholders of Acorda Therapeutics. Other authors report no conflicts of interest.
References
- 1.Burnett AL, Aus G, Canby-Hagino ED, Cookson MS, D’Amico AV, Dmochowski RR, Eton DT, Forman JD, Goldenberg SL, Hernandez J, Higano CS, Kraus S, Liebert M, Moul JW, Tangen C, Thrasher JB, Thompson I. Erectile function outcome reporting after clinically localized prostate cancer treatment. J Urol. 2007;178:597–601. doi: 10.1016/j.juro.2007.03.140. [DOI] [PubMed] [Google Scholar]
- 2.Burnett AL. Erectile Function Outcomes in the Current Era of Anatomic Nerve-Sparing Radical Prostatectomy. Rev Urol. 2006;8:47–53. [PMC free article] [PubMed] [Google Scholar]
- 3.Keast JR. Plasticity of pelvic autonomic ganglia and urogenital innervation. Int Rev Cytol. 2006;248:141–208. doi: 10.1016/S0074-7696(06)48003-7. [DOI] [PubMed] [Google Scholar]
- 4.Schaumburg HH, Zotova E, Cannella B, Raine CS, Arezzo J, Tar M, Melman A. Structural and functional investigations of the murine cavernosal nerve: a model system for serial spatio-temporal study of autonomic neuropathy. BJU Int. 2007;99:916–924. doi: 10.1111/j.1464-410X.2006.06726.x. [DOI] [PubMed] [Google Scholar]
- 5.Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- 6.Facio F, Jr, Burnett AL. Penile rehabilitation and neuromodulation. Scientific World Journal. 2009;9:652–664. doi: 10.1100/tsw.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bella AJ, Lin G, Cagiannos I, Lue TF. Emerging neuromodulatory molecules for the treatment of neurogenic erectile dysfunction caused by cavernous nerve injury. Asian J Androl. 2008;10:54–59. doi: 10.1111/j.1745-7262.2008.00368.x. [DOI] [PubMed] [Google Scholar]
- 8.Podlasek CA. Sonic hedgehog, apoptosis, and the penis. J Sex Med. 2009;6 (Suppl 3):334–339. doi: 10.1111/j.1743-6109.2008.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnett AL, Lue TF. Neuromodulatory therapy to improve erectile function recovery outcomes after pelvic surgery. J Urol. 2006;176:882–887. doi: 10.1016/j.juro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Mulhall JP. Exploring the potential role of neuromodulatory drugs in radical prostatectomy patients. J Androl. 2009;30:377–383. doi: 10.2164/jandrol.108.006866. [DOI] [PubMed] [Google Scholar]
- 11.Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 12.Gambarotta G, Fregnan F, Gnavi S, Perroteau I. Neuregulin 1 role in Schwann cell regulation and potential applications to promote peripheral nerve regeneration. Int Rev Neurobiol. 2013;108:223–256. doi: 10.1016/B978-0-12-410499-0.00009-5. [DOI] [PubMed] [Google Scholar]
- 13.Vullhorst D, Neddens J, Karavanova I, Tricoire L, Petralia RS, McBain CJ, Buonanno A. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009;29:12255–12264. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell KS, Stern DF, Polverini PJ, Bender JR. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol. 1999;277:H2205–H2211. doi: 10.1152/ajpheart.1999.277.6.H2205. [DOI] [PubMed] [Google Scholar]
- 15.Clement CM, Thomas LK, Mou Y, Croslan DR, Gibbons GH, Ford BD. Neuregulin-1 attenuates neointimal formation following vascular injury and inhibits the proliferation of vascular smooth muscle cells. J Vasc Res. 2007;44:303–312. doi: 10.1159/000101776. [DOI] [PubMed] [Google Scholar]
- 16.Hill MF, Patel AV, Murphy A, Smith HM, Galindo CL, Pentassuglia L, Peng X, Lenneman CG, Odiete O, Friedman DB, Kronenberg MW, Zheng S, Zhao Z, Song Y, Harrell FE, Jr, Srinivas M, Ganguly A, Iaci J, Parry TJ, Caggiano AO, Sawyer DB. Intravenous glial growth factor 2 (GGF2) isoform of neuregulin-1β improves left ventricular function, gene and protein expression in rats after myocardial infarction. PLoS One. 2013;8(2):e55741. doi: 10.1371/journal.pone.0055741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fricker FR, Lago N, Balarajah S, Tsantoulas C, Tanna S, Zhu N, Fageiry SK, Jenkins M, Garratt AN, Birchmeier C, Bennett DL. Axonally derived neuregulin-1 is required for remyelination and regeneration after nerve injury in adulthood. J Neurosci. 2011;31:3225–3233. doi: 10.1523/JNEUROSCI.2568-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bella AJ, Hayashi N, Carrion RE, Price R, Lue TF. FK1706 enhances the recovery of erectile function following bilateral cavernous nerve crush injury in the rat. J Sex Med. 2007;4:341–346. doi: 10.1111/j.1743-6109.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- 19.Lagoda G, Sezen SF, Burnett AL. FK506 and rapamycin neuroprotect erection and involve different immunophilins in a rat model of cavernous nerve injury. J Sex Med. 2009;6:1914–1923. doi: 10.1111/j.1743-6109.2009.01293.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen LE, Liu K, Seaber AV, Katragadda S, Kirk C, Urbaniak JR. Recombinant human glial growth factor 2 (rhGGF2) improves functional recovery of crushed peripheral nerve (a double-blind study) Neurochem Int. 1998;33:341–351. doi: 10.1016/s0197-0186(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 21.ter Laak MP, Hamers FP, Kirk CJ, Gispen WH. rhGGF2 protects against cisplatin-induced neuropathy in the rat. J Neurosci Res. 2000;60:237–244. doi: 10.1002/(SICI)1097-4547(20000415)60:2<237::AID-JNR13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Bella AJ, Fandel TM, Tantiwongse K, Brant WO, Klein R, Garcia CA, Lue TF. Neurturin enhances the recovery of erectile function following bilateral cavernous nerve crush injury in the rat. J Brachial Plex Peripher Nerve Inj. 2007;2:5. doi: 10.1186/1749-7221-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagoda G, Xie Y, Sezen SF, Hurt KJ, Liu L, Musicki B, Burnett AL. FK506 neuroprotection after cavernous nerve injury is mediated by thioredoxin and glutathione redox systems. J Sex Med. 2011;8:3325. doi: 10.1111/j.1743-6109.2011.02500.x. –3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashita S, Kato R, Kobayashi K, Hisasue S, Arai Y, Tsukamoto T. Inhibition of interleukin-6 attenuates erectile dysfunction in a rat model of nerve-sparing radical prostatectomy. J Sex Med. 2011;8:1957–1964. doi: 10.1111/j.1743-6109.2011.02283.x. [DOI] [PubMed] [Google Scholar]
- 25.Allaf ME, Hoke A, Burnett AL. Erythropoetin promotes the recovery of erectile function following cavernous nerve injury. J Urol. 2005;174:2060–2064. doi: 10.1097/01.ju.0000176808.94610.dd. [DOI] [PubMed] [Google Scholar]
- 26.Valentine H, Chen Y, Guo Y, McCormick J, Wu Y, Sezen SF, Hoke A, Burnett AL, Stener JP. Neuroimmunophilin ligands protect cavernous nerves after crush injury in the rat: new experimental paradigms. Eur Urol. 2007;51:1724–1731. doi: 10.1016/j.eururo.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen JO. Stereology of nerve cross sections. J Neurosci Methods. 1998;85:107–118. doi: 10.1016/s0165-0270(98)00129-0. [DOI] [PubMed] [Google Scholar]
- 28.Kato R, Wolfe D, Coyle CH, Wechuck JB, Tyagi P, Tsukamoto T, Nelson JB, Glorioso JC, Chancellor MB, Yoshimura N. Herpes simplex virus vector-mediated delivery of neurturin rescues erectile dysfunction of cavernous nerve injury. Gene Ther. 2009;16:26–33. doi: 10.1038/gt.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuura S, Obara T, Tsuchiya N, Suzuki Y, Habuchi T. Cavernous nerve regeneration by biodegradable alginate gel sponge sheet placement without sutures. Urology. 2006;68:1366–1371. doi: 10.1016/j.urology.2006.09.051. [DOI] [PubMed] [Google Scholar]
- 30.Hoke A, Ho T, Crawford TO, LeBel C, Hilt D, Griffin JW. Glial cell line-derived neurotrophic factor alters axon Schwann cell units and promotes myelination in unmyelinated nerve fibers. J Neurosci. 2003;23:561–567. doi: 10.1523/JNEUROSCI.23-02-00561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snodgrass-Belt P, Gilbert JL, Davis FC. Central administration of transforming growth factor-alpha and neuregulin-1 suppress active behaviors and cause weight loss in hamsters. Brain Res. 2005;1038:171–182. doi: 10.1016/j.brainres.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 32.Canguven O, Burnett A. Cavernous nerve injury using rodent animal models. J Sex Med. 2008;5:1776–1785. doi: 10.1111/j.1743-6109.2008.00955.x. [DOI] [PubMed] [Google Scholar]
- 33.Sezen SF, Hoke A, Burnett AL, Snyder SH. Immunophilin ligand FK506 is neuroprotective for penile innervation. Nat Med. 2001;7:1073–1074. doi: 10.1038/nm1001-1073. [DOI] [PubMed] [Google Scholar]
- 34.Burnett AL, Becker RE. Immunophilin ligands promote penile neurogenesis and erection recovery after cavernous nerve injury. Journal of Urology. 2004;171:495–500. doi: 10.1097/01.ju.0000089775.88825.ec. [DOI] [PubMed] [Google Scholar]
- 35.Bhatheja K, Field J. Schwann cells: origins and role in axonal maintenance and regeneration. Int J Biochem Cell Biol. 2006;38:1995–1999. doi: 10.1016/j.biocel.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Scheib J, Hoke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013;9:668–676. doi: 10.1038/nrneurol.2013.227. [DOI] [PubMed] [Google Scholar]
- 37.Yildiz M, Karlidag T, Yalcin S, Ozogul C, Keles E, Alpay HC, Yanilmaz M. Efficacy of glial growth factor and nerve growth factor on the recovery of traumatic facial paralysis. Eur Arch Otorhinolaryngol. 2011;268:1127–1133. doi: 10.1007/s00405-011-1492-3. [DOI] [PubMed] [Google Scholar]
- 38.Fricker FR, Bennett DL. The role of neuregulin-1 in the response to nerve injury. Future Neurol. 2011;6:809–822. doi: 10.2217/fnl.11.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wakatsuki S, Araki T, Sehara-Fujisawa A. Neuregulin-1/glial growth factor stimulates Schwann cell migration by inducing α5 β1 integrin-ErbB2-focal adhesion kinase complex formation. Genes Cells. 2014;19:66–77. doi: 10.1111/gtc.12108. [DOI] [PubMed] [Google Scholar]
- 40.Chausovsky A, Tsarfaty I, Kam Z, Yarden Y, Geiger B, Bershadsky AD. Morphogenetic effects of neuregulin (neu differentiation factor) in cultured epithelial cells. Mol Biol Cell. 1998;9:3195–3209. doi: 10.1091/mbc.9.11.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]