Abstract
Type 1 diabetes is an autoimmune-mediated disease resulting in the destruction of insulin-secreting pancreatic β-cells. Transplantation of insulin-producing islets is a viable treatment to restore euglycemia in Type 1 diabetics, however, the clinical application remains limited due to the use of toxic immunosuppressive therapies to prevent immune-mediated rejection. We present a nanothin polymer material with dual antioxidant and immunosuppressive properties capable of modulating both innate and adaptive immune responses crucial for transplantation outcome. Through the use of hollow microparticles (capsules) comprising of hydrogen-bonded multilayers of natural polyphenol (tannic acid) with poly(N-vinylpyrrolidone) (TA/PVPON) and with poly(N-vinylcaprolactam) (TA/PVCL), pro-inflammatory reactive oxygen and nitrogen species are efficiently dissipated and the production of IFN-γ and TNF-α pro-inflammatory cytokines are attenuated by cognate antigen-stimulated autoreactive CD4+ T cells. Our results provide evidence that TA-containing capsules are efficacious in immunomodulation and may provide physical transplant protection and prevent diabetogenic autoreactive T cell responses. Future studies will determine if xeno- and allotransplantation with (TA/PVPON)- or (TA/PVCL)-coated pancreatic islets will decrease the risk of graft rejection due to attenuation of oxidative stress and IFN-γ, and restore euglycemia in Type 1 diabetics.
1. Introduction
Type 1 diabetes (T1D) is an autoimmune-mediated life-threatening disease resulting in the destruction of insulin-secreting pancreatic β-cells by islet-infiltrating leukocytes, including both direct (cytotoxic T cell-mediated) and indirect (cytokine and reactive oxygen species (ROS)-mediated) mechanisms. While these causes of β-cell destruction are fundamentally different, both involve the generation of cytotoxic ROS.[i–ii,iii,iv] In T1D, self-tolerance against insulin-producing β-cells is compromised, as autoreactive CD4+ and CD8+ T cells synthesize pro-inflammatory cytokines interferon-γ (IFN-γ) and tumor necrosis factor (TNF-α) and cytotoxic mechanisms including Fas and perforin to destroy insulin-producing β-cells.[v,vi] Blocking IFN-γ by a soluble non-immunogenic form of the IFN-γ receptor has been shown to improve the disease conditions while in presence of IL-12p70, a promoter of T helper 1 (Th1) cell differentiation, the disease conditions are aggravated.[vii,viii] In addition, autoreactive CD4+ T cells may recruit macrophages to the islet and contribute to pancreatic β-cell destruction by releasing high levels of IL-1β, TNF-α, and ROS.[ix–x,xi,xii] In turn, ROS can further exacerbate β-cell damage by functioning as a signaling molecule to induce the activation of pro-inflammatory cytokine signaling pathways in macrophages and dendritic cells.[iii]
As a result of insulin deficiency in T1D, numerous complications including cardiovascular disease, nephropathy and retinopathy may ensue and diminish the quality of life.[xiii] In this case, the use of exogenous insulin is vital for maintaining normal glucose levels in blood. Transplantation of insulin-producing cells or pancreatic islets is considered the most reliable strategy to achieve in vivo glucose control and prevent the devastating complications associated with T1D.[xiv] However, despite early successes, the clinical application of islet transplantation remains limited due to challenges associated with islet integrity and function after isolation from the pancreata of cadaveric donors and the use of toxic immunosuppressive therapies to protect donor islets from immune-mediated rejection.[xv] These challenges have inspired the development of islet encapsulation[xvi–xvii,xviii,xix] and islet surface modification[xx–xxi,xxii,xxiii] strategies to stabilize islet morphology, functionality, and ensure transplant survival without or with minimal immunosuppressive therapy.[xxiv] Recently, we have reported on a nanoscale protective coating of individual islets using a hydrogen-bonded layer-by-layer (LbL) assembly of tannic acid (TA) and poly(N-vinylpyrrolidone) (PVPON).[xxv] The (TA/ PVPON) multilayer-coated islets were stable for 7 days in vitro, non-toxic, and displayed an elevated insulin stimulation index in vitro culture for 96 hours as compared to uncoated islets.[xxv]
TA is a natural polyphenolic antioxidant and has been used in fabrication of multilayer films, capsules, and cell modification coatings of biomedical relevance.[xxvi,xxvii,xxviii,xxix,xxx,xxxi,xxxii,xxxiv,xxxv] The antioxidant activities of TA[xxxvi–xxxvii,xxxviii,xxxix,xl] may be beneficial for TA-containing protective coatings since most inflammatory processes are associated with oxidative stress due to enhanced ROS synthesis and/or a decrease in antioxidant activities.[75,76] ROS are highly reactive oxygen derivatives which include the superoxide radical (O2.-), the hydroxyl radical (.OH), and hydrogen peroxide (H2O2); those along with reactive nitrogen species such as nitric oxide (NO) can function as signaling molecules for immune activation.[77] Lvov, et al., showed that antioxidant activity of TA is preserved in TA/polycation multilayers. [xli] He, et al., reported that antioxidant stability of a drug formulation was improved when poly(triethylene glycol methyl acrylate-co-tocopheryl acrylate) complexed with TA was used as a potential antioxidant pro-drug to enable localized neuroprotection.[xlii] Recently, De Geest and co-workers encapsulated tumor associated antigen by conjugating cancer cells with (TA/PVPON) multilayers to be used for anticancer immunotherapy.[xliii] The same group showed that (TA/PVPON) microparticles with encapsulated vaccine protein antigens were effectively internalized by dendritic cells in vitro and promoted the antigen-specific humoral and cellular immune responses in vivo as potential vaccine carriers.[xliv]
Despite the intriguing potential of TA, little is known about immunomodulatory and antioxidant capability of TA-based materials to provide islet protection and prevent autoreactive T cell responses, crucial for successful transplantation. In the process of human islet isolation, β-cells in isolated pancreatic islets can be impaired by oxidative stress[xlv] because of the reduced levels of their inherent antioxidant protection, which can lead to imbalanced levels of ROS. Activation of macrophages and T cells by ROS followed by the secretion of pro-inflammatory cytokines can significantly contribute to the progression of immune system activation and inflammation[xlvi] and potentially lead to functional impairment of the islet transplant. Typically, immunomodulatory compounds employed in different models of oxidative stress injuries are directly injected into tissues. In one example, isolated islets demonstrated higher survival after their transplantation in mice when cultured in the presence of Mn (III) porphyrin-based catalytic antioxidants suggesting that the modulation of oxidative stress can help in better islet protection.[xlvii] There are only a few studies on functionalization of coatings with the active compounds, mostly limited to conjugation to islets or copolymerization into gels on the islet surfaces.[xlviii,xlix]
Our group recently demonstrated that (TA/PVPON) multilayer coatings suppressed immune cell-mediated proinflammatory cytokine synthesis.[xxv] However, the mechanism through which TA-based coating exhibits immunomodulatory activity remains unclear. In the current study we explore the capability of TA-containing multilayer capsules (also known as shells) as immune mediators capable of modulating both innate and adaptive immune responses important for transplantation outcome. We determined the efficacy of various types of hydrogen-bonded multilayers of tannic acid to scavenge reactive oxygen and nitrogen species, as well as the potential of TA-containing (PVPON/TA) or (poly(N-vinylcaprolactam)/TA) (PVCL/TA) hydrogen-bonded protective shells to dampen pro-inflammatory cytokine production from diabetogenic autoreactive T cells. The effects of capsule thickness, composition, and outer layer are studied under various stimulation type and time. To the best of our knowledge this is the only example of a nanothin material with dual immunomodulatory and antioxidant functionality capable of modulating both innate and adaptive immune responses, crucial for allo- and xenotransplantation.
2. Results and Discussion
Scavenging of ROS by the TA-containing shells
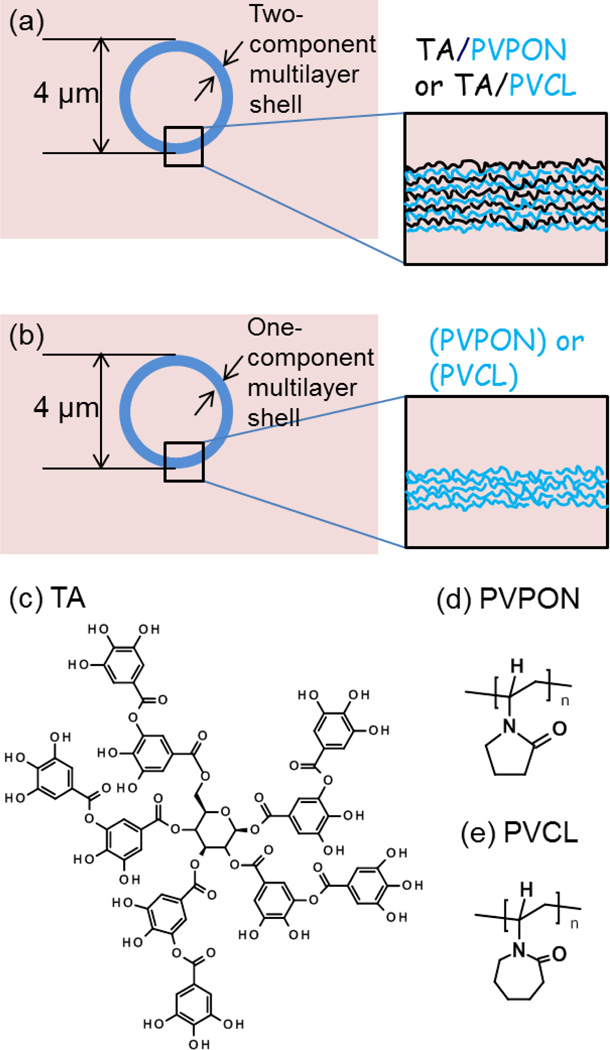
We explored the antioxidant capability of the hydrogen-bonded coatings of TA by determining the ability of PVPON/TA or PVCL/TA hydrogen-bonded shells to dissipate ROS synthesis. For that, the certain concentrations of the corresponding hydrogen-bonded multilayer capsules of (neutral polymer/TA) were prepared as hollow replicas of 4-µm silica particles coated with the corresponding multilayers (Fig. 1a) as reported previously[xxv] and tested using respiratory burst assay. As control, TA-free hollow multilayer capsules made from either PVPON or PVCL multilayers were used (Fig. 1b).[lxx,l]
Figure 1.
(a) TA-containing multilayer capsules of (TA/PVPON)n, or (TA/PVCL)n were prepared via LbL assembly of TA and PVPON (or PCVCL) on spherical silica particles of 4 µm (b) TA-free capsules of (PVPON)n or (PVCL)n multilayers. (c, d, e) Chemical structures of tannic acid (TA), poly(N-vinylpyrrolidone (PVPON), and poly(N-vinylcaprolactam) (PVCL).
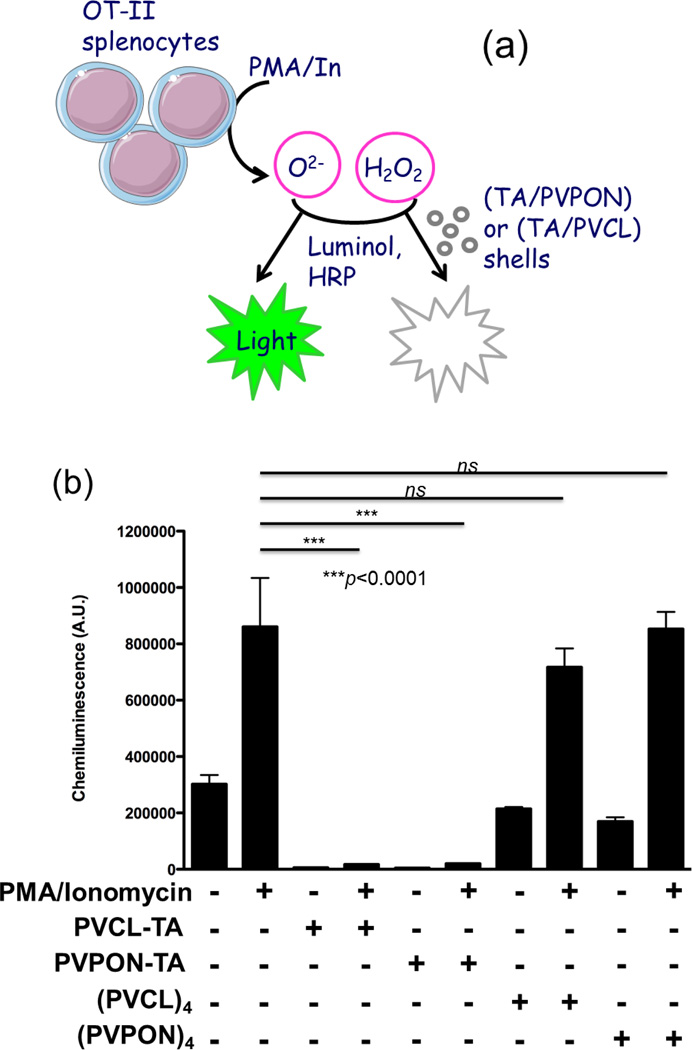
Using a luminol oxidation assay, we observed total suppression of ROS synthesis in the presence of TA-containing shells (Fig. 2). The experiments were based on the production of superoxide (O2.-) and hydrogen peroxide (H2O2) after stimulation of OT-II splenic T cells (splenocytes) with phorbol 12-myristate 13-acetate (PMA) and ionomycin (In). PMA/In-stimulated splenocytes rapidly released O2.- and H2O2 to facilitate the oxidation of luminol in the presence of horseradish peroxidase (HRP) and emission of chemiluminescence (Fig. 2a).[li] The resultant luminol-dependent chemiluminescence was measured in the presence (+) or absence (−) of TA-containing, (PVPON/TA)4 and (PVCL/TA)4, or control TA-free, (PVPON)4 and (PVCL)4 multilayer shells. Chemiluminescence increased almost 3-fold compared to background when splenocytes were stimulated (Fig. 2b). In drastic contrast, ROS production was suppressed in the presence of (PVPON/TA)4 and (PVCL/TA)4 shells as chemiluminescence did not increase after PMA/In stimulation of the T cells. Importantly, splenocytes incubated with TA-free (PVPON)4 or (PVCL)4 multilayer shells did not exhibit a significant decrease in chemiluminescence compared to the control group. These data suggest that the high antioxidant activity of (PVPON/TA) and (PVCL/TA) capsules is due to the presence of TA within the shells. Apparently, despite TA H-bonding with PVPON or PVCL, there are still phenolic groups available which are liberated from H-bonding at pH=7.4 and capable of scavenging ROS without compromising stability of the multilayer shell.[lii] We found that the synthesis of free radicals detected by the luminol oxidation at 20 minutes post-treatment with TA-containing multilayer shells and beyond an hour was undetectable. As we have demonstrated previously, scavenging of innate immune-derived free radicals at these early time points will have a profound inhibitory effect on the differentiation and maturation of antigen-specific T cells.[iv,ix,lx]
Figure 2.
(a) Scavenging of ROS synthesis by the TA-based hydrogen-bonded coatings. (b) Luminol oxidation assay with OT-II splenocytes stimulated for 20 minutes with 100ng/mL PMA and 1µg/mL ionomycin (In) in the presence (+) or absence (−) of TA-containing hydrogen-bonded shells (PVCL/TA)4, (PVPON/TA)4, or TA-free (PVCL)4, and (PVPON)4 multilayer shells. Data shown is representative of 3 independent experiments performed with at least triplicates for each sample. *** p<0.001, ns not significant.
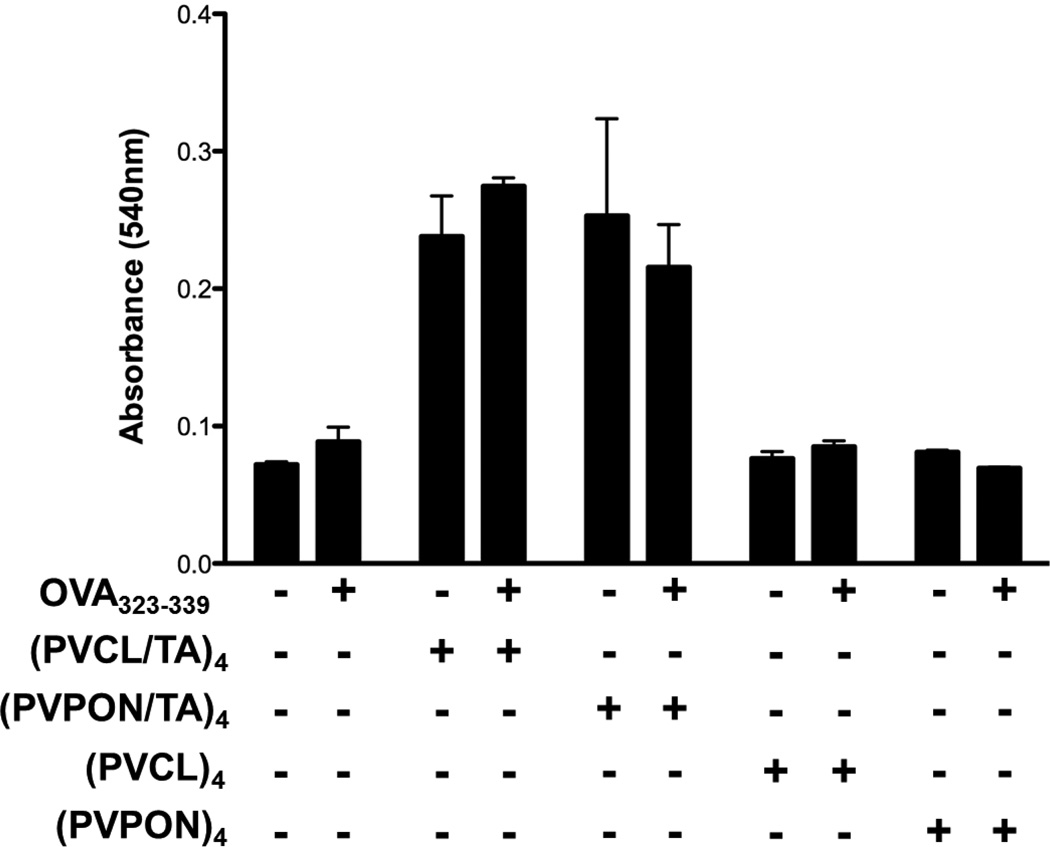
We also found that neither the TA-containing nor TA-free multilayer shells compromised the viability of OT-II splenocytes. For that, MTT cell viability assay was performed with OT-II splenocytes stimulated with 1µM OVA323–339 in the presence or absence of TA-containing hydrogen-bonded (PVCL/TA)4 and (PVPON/TA)4 capsules or TA-free multilayer shells of (PVCL)4, (PVPON)4 for 24 hours (Fig. 3). We did not observe any significant decrease in absorbance from the cells in the presence of shells compared to that in the case of the activated splenocytes alone. These results suggest that the cell viability was not compromised, and the decrease in chemiluminescence resulted from the antioxidant activity of the TA-containing hydrogen-bonded shells (Fig. 2). The increase in absorbance by splenocytes treated with TA-based shells may be attributed to an increase in overall cell viability as compared to shells lacking TA. Since TA is an antioxidant, TA-containing shells may alter the redox state of antigen-stimulated T cells and enhance proliferation and survival (Fig. 3).
Figure 3.
MTT cell viability assay was performed with OT-II splenocytes stimulated with 1µM OVA323–339 in the presence (+) or absence (−) of TA-containing hydrogen-bonded (PVCL/TA)4, and (PVPON/TA)4, or TA-free (PVCL)4 and (PVPON)4 multilayer capsules for 24 hours. Data shown is representative of 3 independent experiments performed with at least triplicates for each sample.
Diminishing of nitrite synthesis by TA- containing shells
Nitric oxide has been established to be the primary mediator for cytokine–induced islet damage as well as it can lead to inhibition of insulin release from islet β-cells.[liii] To mediate physiological signaling, nitric oxide and other reactive nitrogen species can be produced from NO2− through its reduction by all mammalian reductases under hypoxia and acidosis.[liv] Thus, in addition to dissipating ROS synthesis, the ability of TA-containing hydrogen-bonded shells to scavenge reactive nitrogen species was examined. BDC-2.5 splenocytes (5×105) were stimulated with 1 µM cognate BDC-2.5 mimotope for 96 hours and the nitrite levels in tissue culture supernatants were assayed by the Greiss assay.
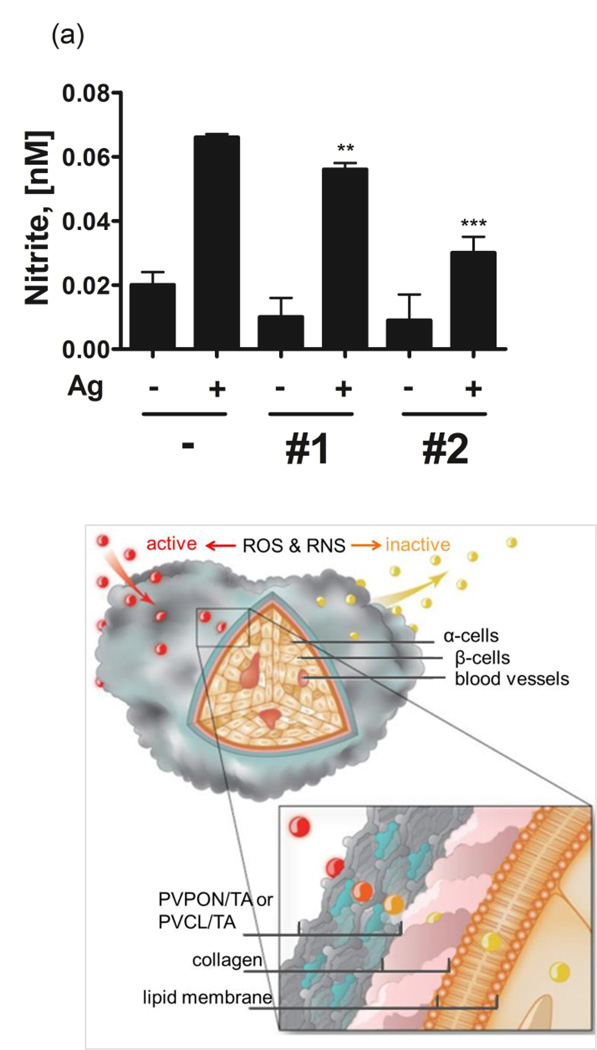
Figure 4a demonstrates that a 3-fold increase in NO2− concentration from 0.02 nM to 0.067 nM was observed in the culture supernatants of the splenocytes alone in comparison to unstimulated controls. Remarkably, the stimulation of BDC-2.5 splenocytes with their cognate mimotope in the presence of 5×106 (PVCL/TA)5 and (PVPON/TA)5 hydrogen-bonded shells for 96 hours resulted in diminished nitrite levels by 2-fold, from 0.067 nM to 0.029 nM for (PVCL/TA)5, and by 1.2-fold, from 0.067 nM to 0.057 nM, for (PVPON/TA)5 after the 96-h co-treatment (Fig.4a). The more efficient, almost 2-fold, dissipation of nitrite levels in culture solutions by (PVCL/TA) shells may be attributed to a larger shell thickness of the system compared to that of (PVPON/TA). PVCL is a hydrophobic homologue of PVPON with two additional methylene groups in the lactam ring,[lv] therefore, the levels of TA deposited within one bilayer can be controlled by varying hydrophobicity of the neutral polymer assembled with TA allowing for obtaining thicker PVCL/TA bilayers compared to PVPON/TA bilayers. Thus, as reported previously, the average bilayer thickness of (PVPON/TA) and (PVCL/TA) capsule walls prepared under similar conditions were 2.2±0.2 nm and 4.2±0.1 nm, respectively.[lvi] Subsequently, our results on nitrite dissipation by TA-containing hydrogen-bonded shells are in good agreement with the thickness data for those capsule systems implying that 2-fold increased amount of TA in PVCL/TA capsules compared to that in PVPON/TA resulted in a 2-fold decrease of nitrite levels (Fig. 4a). Based on the results, we calculated the amount of TA assembled within the hydrogen-bonded coating which is necessary to dissipate 1 nM of nitrite to be ~69 µg. Given 1 mg m−2 adsorbed amount of polymer per 1 nm film thickness,[lvii] total amount of TA adsorbed within a spherical (TA/polymer)n shell was obtained as [TA]10−3 g = πntTAd2, in which n is the number of bilayers adsorbed within the multilayer; tTA is an average TA thickness (nm, taken as 50% of the average bilayer thickness[lii]), measured by AFM for the shells; d (m) is the size of the sacrificial spherical template used for capsule preparation (4 µm).
Figure 4.
(a) Tissue culture supernatants from BDC-2.5 splenocytes (5×105) stimulated with 1µM BDC-2.5 mimotope in the presence (+) or absence (−) of 5×106 (PVPON/TA)5 (#1), (PVCL/TA)5 (#2) capsules for 96 hours were assayed for nitrite levels by the Greiss assay. Data shown is representative of 3 independent experiments performed with at least triplicates for each sample . *** p<0.001, ** p<0.005 in comparison to BDC-2.5 mimotope-stimulated samples alone. (b) Schematics of ROS and RNS protection of islets coated by (PVCL/TA) or (PVPON/TA) hydrogen-bonded coatings.
In Figure 4b the protective properties of PVCL/TA and PVPON/TA hydrogen-bonded multilayer coatings against reactive oxygen and nitrogen species are schematically summarized. The scavenging of free radicals by the coating because of the antioxidant properties of TA component can lead to suppression of pro-inflammatory cytokine synthesis via dampening redox-dependent signaling pathways and islet protection. Our results provide evidence that by dissipation of ROS synthesis, TA-based hydrogen-bonded coatings should be able to diminish diabetogenic T cell effector responses as we have previously demonstrated with a superoxide dismutase mimetic compound.[xlviii,lx,lviii]
Inhibition of adaptive immune effector responses with TA- containing shells
We have previously shown that modulating redox status during innate immune activation is efficient in attenuating the synthesis of pro-inflammatory cytokines that can contribute to adaptive immune maturation and rejection of transplanted islets.[ix,lix,lx] Herein, we have studied whether TA-containing hydrogen-bonded coatings could produce a similar modulating effect on the adaptive immune response of autoreactive T cells. The immunomodulatory properties were investigated using three types of pro-inflammatory cytokines (IFN-γ, TNF-α, and IL-2) synthesized by stimulated diabetogenic autoreactive CD4+ T cells.
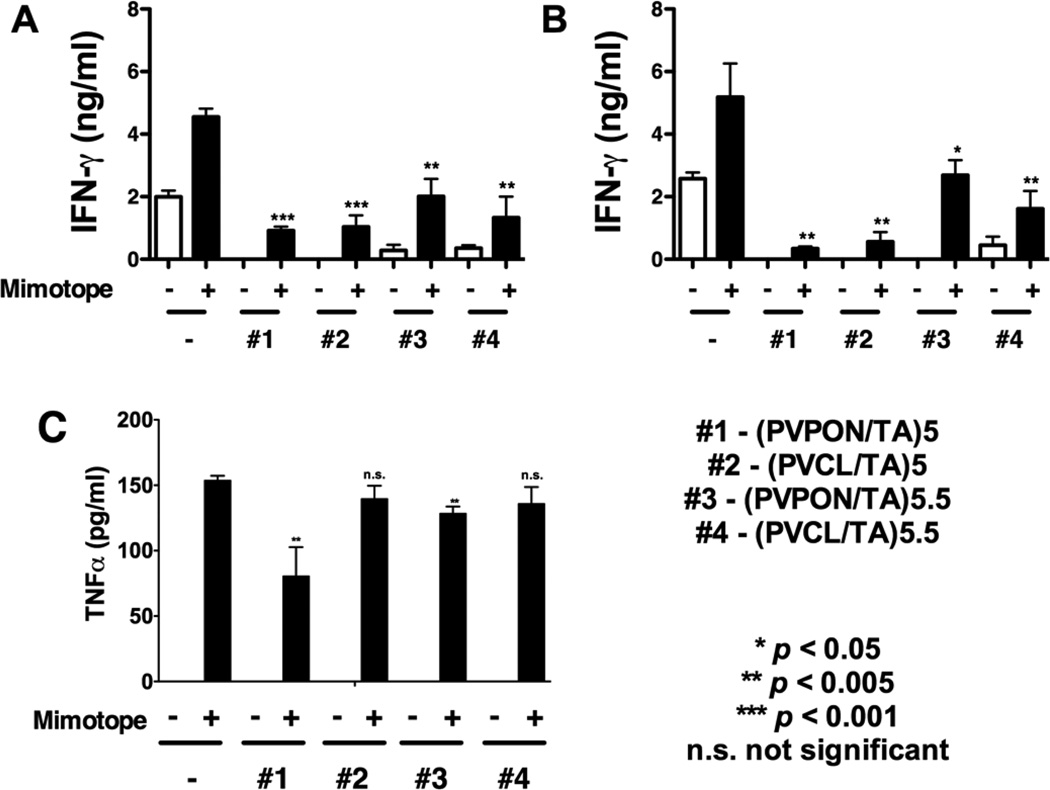
The effect of the coatings on suppression of autoreactive BDC-2.5 CD4+ T cell cytokine responses was examined by measuring IFN-γ released by BDC-2.5 splenocytes after stimulation with their cognate BDC-2.5 mimotope antigenic peptide in the presence (+) or absence (−) of the hollow capsules for 24 and 48 hours. To determine the effect of the coating top layer, the multilayer capsules with TA capping layer labeled as (PVPON/TA)5 or (PVCL/TA)5, and with a neutral capping layer labeled as (PVPON/TA)5.5 or (PVCL/TA)5.5, were prepared and tested.
Figure 5 demonstrates that all TA-based shells were effective in significantly dampening IFN-γ synthesis. For instance, capsule-free BDC-2.5 mimotope stimulation for 24 h resulted in 4.7 ng mL−1 IFN-γ synthesis, while a 4-fold decrease in the cytokine levels was observed for the T cell stimulation in the presence of (PVPON/TA)5 and (PVCL/TA)5 capsules (1 ng mL−1), with a 2.3-fold and 3.4-fold decrease in IFN-γ synthesis in co-culture with (PVPON/TA)5.5 and (PVCL/TA)5.5 capsules respectively (Fig. 5a). The cytokine synthesis attenuation was even more pronounced after 48-h stimulation of the T cells in the presence of TA-containing hydrogen-bonded shells resulting in 14- and 8-fold decrease in synthesis of IFN-γ in the presence of (PVPON/TA)5 and (PVCL/TA)5 capsules, respectively (Fig. 5b). In comparison to TA-capped capsules, the neutral polymer-capped (PVPON/TA)5.5 and (PVCL/TA)5.5 shells were less effective in dampening IFN-γ production showing a 2- and 3-fold decrease in the cytokine synthesis, respectively (Fig. 5b). In addition to a decrease in IFN-γ production, TNF-α levels from BDC-2.5 splenocytes stimulated with the BDC-2.5 mimotope in the presence of TA-based hydrogen-bonded capsules were also decreased in the presence of (PVPON/TA)5 and (PVPON/TA)5.5 capsules (Fig. 5c).
Figure 5.
BDC-2.5 primary recall assay with BDC-2.5 mimotope and TA-containing capsules. Supernatants from BDC-2.5 splenocytes (5×105) stimulated with 1 µM BDC-2.5 mimotope in the presence or absence of 5×106 (PVPON/TA)5 (#1), (PVCL/TA)5 (#2), (PVPON/TA)5.5 (#3), and (PVCL/TA)5.5 (#4) capsules for 24 (a) and 48 hours (b) were assayed for IFN-γ and for TNF-α (c) by ELISA. Data shown is representative of 3 independent experiments performed with at least triplicates for each sample. *** p<0.001, ** p<0.005, * p<0.05, ns - not significant in comparison to BDC-2.5 mimotope-stimulated samples alone.
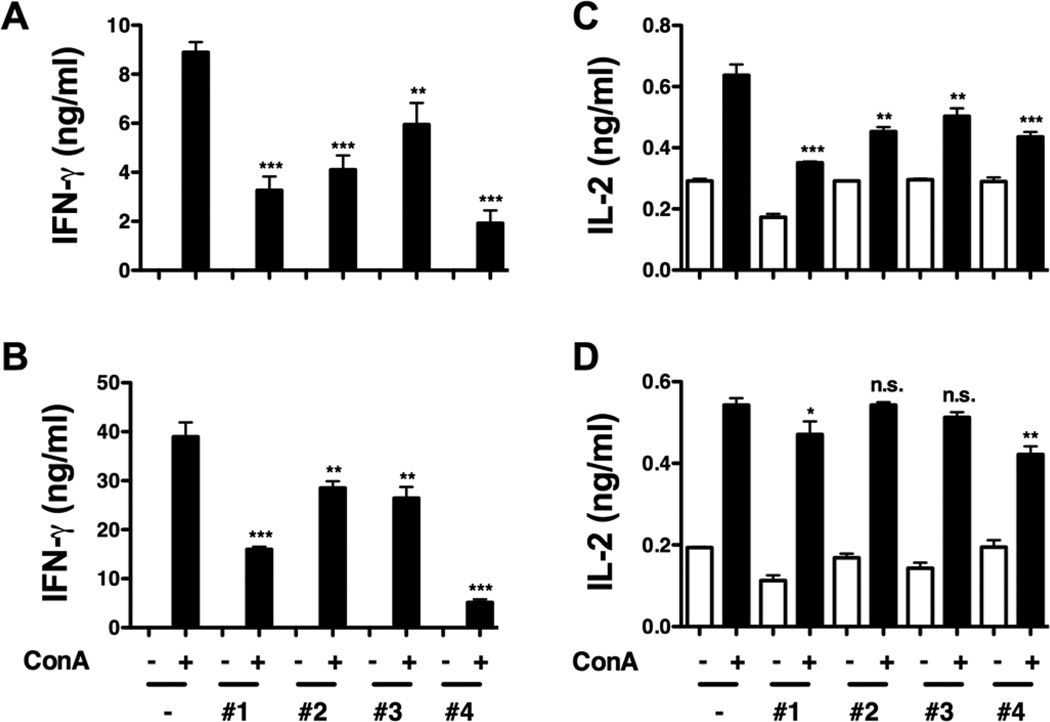
To determine if TA-based shells affect Th1 cytokine responses due to stimulation with the BDC-2.5 mimotope alone, BDC-2.5 splenocytes were stimulated with the mitogen, concanavalin A (ConA), a non-specific inducer of T cell responses, in the presence (+) or absence (−) of the capsules for 24 and 48 hours. The ability of TA-based hydrogen-bonded shells to decrease autoreactive effector T cell responses upon stimulation with ConA or the BDC-2.5 mimotope provides additional evidence that islet encapsulation with the TA-based coating may elicit an immunoprotective effect in the context of islet allo- and xenotransplantation. ConA-stimulation for 24 and 48 hours resulted in a decreased IFN-γ synthesis, similar to what was observed with BDC-2.5 mimotope-stimulated splenocytes. For TA-capped 5-bilayer capsules, (PVPON/TA) system seemed to be more effective than (PVCL/TA) in suppression of the pro-inflammatory cytokine production with 61% versus 53% decrease of IFN-γ levels, respectively, compared to that for the capsule-free stimulation (Fig. 6a). The opposite was observed in the case of the capsules with TA shielded by a layer of neutral polymer with 33% versus 88% decrease in the cytokine synthesis for (PVPON/TA)5.5 and (PVCL/TA)5.5, respectively. Similar results were obtained after 48-h stimulation of the T cells in the presence of the capsules (Fig. 6b).
Figure 6.
Concanavalin A (ConA)-stimulated BDC-2.5 splenocytes treated with TA-containing shells. Supernatants from BDC-2.5 splenocytes (5×105) stimulated with 2.5µg/mL ConA in the presence or absence of 5×106 (PVPON/TA)5 (#1), (PVCL/TA)5 (#2), (PVPON/TA)5.5 (#3), and (PVCL/TA)5.5 (#4) capsules for 24 (a, c) and 48 hours (b, d) were assayed for IFN-γ (a, b) and IL-2 (c, d) synthesis by ELISA. Data shown is representative of 3 independent experiments performed with at least triplicates for each sample. *** p<0.001, ** p<0.005, * p<0.05, ns not significant in comparison to BDC-2.5 ConA-stimulated samples alone.
Next, the synthesis of interleukin-2 (IL-2), a cytokine involved in T cell proliferation, was monitored for 24 and 48 hours. The IL-2 levels decreased from 0.64 ng mL−1 to 0.36 ng mL−1, to 0.45 ng mL−1, to 0.5 ng mL−1, and to 0.44 ng mL−1 when ConA-stimulated BDC-2.5 splenocytes were co-treated with (PVPON/TA)5, (PVCL/TA)5, (PVPON/TA)5.5, and (PVCL/TA)5.5, respectively for 24 hours (Fig. 6c). However, the suppressive effects were transient as IL-2 synthesis was restored to nearly control levels after 48 hours of stimulation (Fig. 6d). This result agrees well with our previous work where no significant change in IL-2 synthesis was observed after 96 hours of T cell stimulation.[xxv]
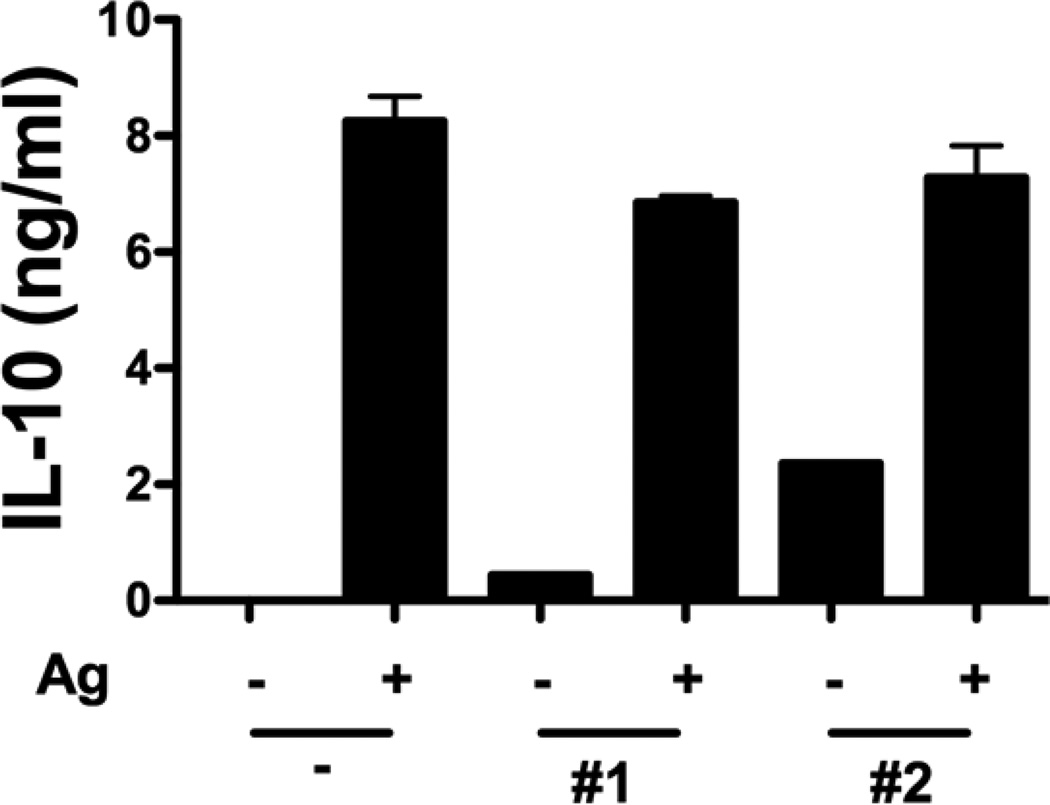
An important question is if the suppressive effects, observed in co-culture of T cells with TA-based shells, were due to an increase in synthesis of anti-inflammatory IL-10 cytokine, a major suppressor of Th1 cytokine synthesis.[lxi] To address the question, co-treatment of mimotope-stimulated BDC-2.5 splenocytes with (PVPON/TA)5, (PVCL/TA)5 shells for 72 hours was performed and no enhancement of IL-10 production was observed (Fig. 7). These results confirmed that the suppression of Th1 effector responses discussed above were indeed because of the TA-based hydrogen-bonded coatings. The mechanisms through which TA-based capsules affect T cell cytokine synthesis remain to be yet fully elucidated. However, as reported previously, green tee polyphenols, e. g., epigallocatechin-3-gallate, were shown to inhibit both IFN-γ and IL-2 production by B6 spleen cells beginning at concentration > 10 µg mL−1 through inhibition of intracellular signals involved in T cell activation and differentiation into Th1 effector cells.[lxii,lxiii] In our case, the total concentration of TA assembled within the shells at which the inhibition of IFN-γ was observed for (PVPON/TA) shells was 13 µg mL−1 which is similar to the reported value.
Figure 7.
Production of IL-10 in tissue culture supernatants after 72-hour stimulation in the absence () or presence (+) of 5×106 (PVPON/TA)5 (#1), (PVCL/TA)5 (#2) capsules as detected by ELISA. Data shown is representative of 3 independent experiments performed with at least triplicates for each sample.
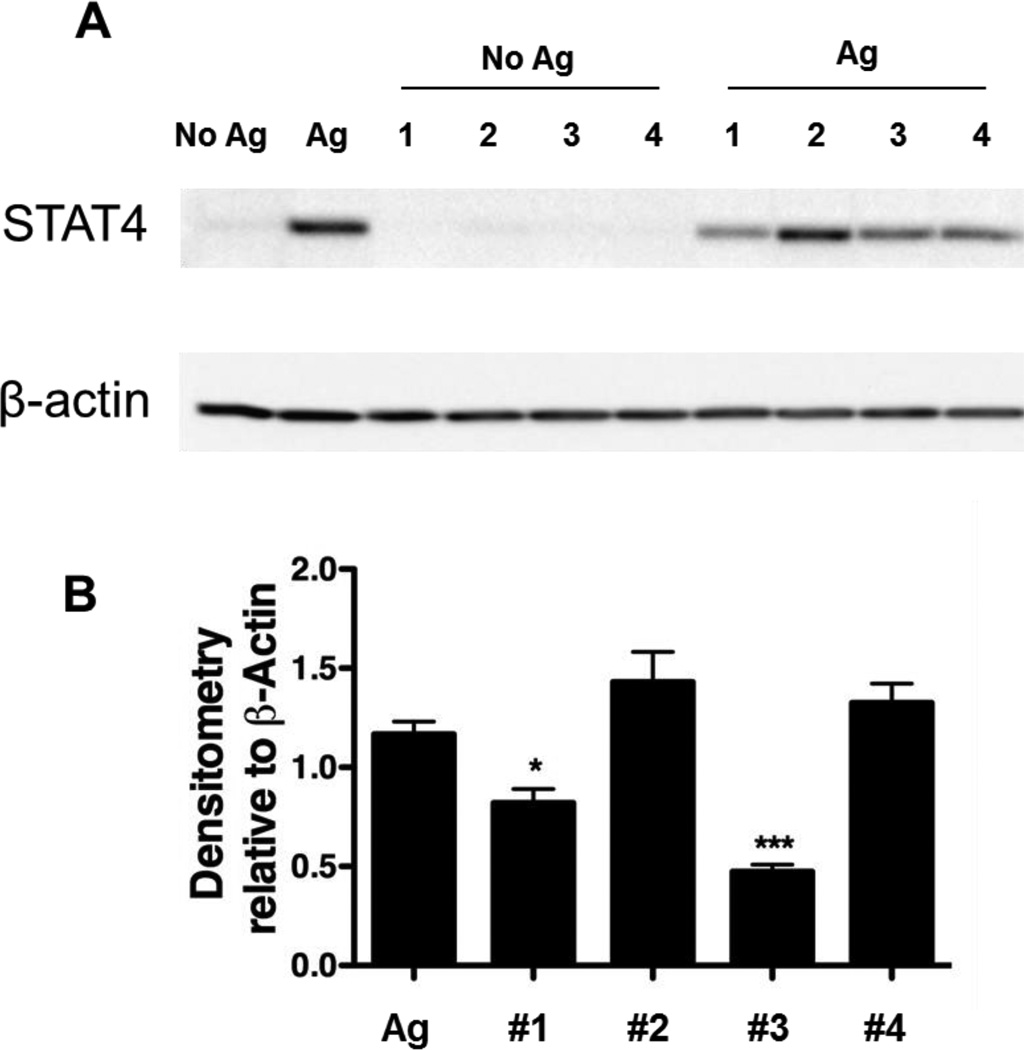
The STAT4 signaling pathway is important in the differentiation of CD4+ T cells producing Th1 cytokine (IFN-γ) responses. STAT4 functions as a transcription factor to induce Ifng mRNA accumulation. Thus, to define the mechanism for the observed decrease in Th1 cytokine synthesis (IFN-γ) mediated by TA-based shells on autoreactive CD4+ T cells, the expression of the STAT4 signaling pathway for IFN-γ synthesis was examined by Western blot analysis using β-actin as a loading control. Whole cell lysates from 5×105 BDC-2.5 splenocytes stimulated with 1 µM BDC-2.5 mimotope in the presence or absence of 5×106 shells for 48 hours were probed in a Western blot for STAT4 and β-actin expression. As shown in Figure 8a, mimotope stimulation of BDC-2.5 splenocytes alone resulted in an increase in STAT4 protein expression while no STAT4 expression was observed without T cell stimulation in the presence of TA-based hydrogen-bonded capsules. The co-stimulation of the splenocytes with (PVPON/TA)5 and (PVPON/TA)5.5 capsules elicited a significant decrease in STAT4 protein expression as demonstrated by densitometry analysis with β-actin expression (Fig. 8) while no inhibition on the STAT4 expression was seen in the case of PVCL/TA capsules. Our data demonstrates that the inhibition of the STAT4 signaling pathway (Fig. 8) that is involved in transcriptional regulation of Ifng[lxiv,lxv,lxvi] is mediated by (PVPON/TA)5 and (PVPON/TA)5.5 capsules, but not by PVCL/TA capsules. We did not observe a decrease in the levels of IL-10 synthesis with TA-containing capsules (Fig. 7) and subsequently, this provides evidence that inhibition of pro-inflammatory IFN-γ secretion is occurring at the transcriptional level. These results suggest that there is an ensemble of various paths for IFN-γ production suppression observed in Figures 5 and 6 that are affected by TA-based hydrogen-bonded capsules and modulating STAT4 expression is among those. Previously described, polyphenolic antioxidants quercitin and epigallocathechin-3-gallate inhibited the STAT4 signaling pathway in purified T lymphocytes, and ameliorated the autoimmune disease, experimental autoimmune encephalomyelitis.[lxvii] The suppression of IL-2 by quercitin was also demonstrated in Th1 cells, however, the mechanism of such repression was found to be different from IFN-γ suppression.[lxviii]
Figure 8.
BDC-2.5 primary recall assay with BDC-2.5 mimotope and 5×106 (PVPON/TA)5 (#1), (PVCL/TA)5 (#2), (PVPON/TA)5.5 (#3), and (PVCL/TA)5.5 (#4) capsules. Densitometry statistics for Western blotting represent the average of 3 experiments. *p<0.05 and ***p<0.001 in comparison to BDC-2.5 mimotope-stimulated samples alone.
3. Conclusions
We showed that (TA/PVPON) and (TA/PVCL) multilayers display efficient antioxidant and immunomodulatory properties by scavenging free radicals and suppressing the synthesis of IFN-γ and TNF-α pro-inflammatory cytokines, respectively. IFN-γ synthesis from autoreactive CD4+ T cells was attenuated 14-fold in the presence (TA/PVPON)5 capsules and 8-fold in the presence of (TA/PVCL)5 capsules. The total concentration of TA assembled within the shells at which the inhibition of IFN-γ was observed for (PVPON/TA) shells was 13 µg mL−1. In comparison to TA-capped capsules, the neutral polymer-capped (PVPON/TA)5.5 and (PVCL/TA)5.5 shells were less effective in dampening IFN-γ production showing a 2- and 3-fold decrease in the cytokine synthesis, respectively. We suggest that the free radical scavenging activity of the capsules is because of the antioxidant properties of TA component. Dissipation of ROS synthesis diminishes diabetogenic T cell effector responses via dampening the redox-dependent STAT4 signaling pathway involved in IFN-γ synthesis. Overall, our study demonstrates that hydrogen-bonded LbL technology when applied for coating individual, living pancreatic islets have the potential to control and regulate the production of pro-inflammatory cytokines in vicinity of the islet graft coated with TA-containing films. Transplantation of (PVPON/TA)- or (PVCL/TA)-coated islets may decrease the risk of xeno- and allograft rejection due to the attenuation of oxidative stress and pro-inflammatory cytokine synthesis, and ultimately, enable Type 1 diabetics a lifestyle independent of exogenous insulin administration.
4. Experimental Section
Materials
Poly(N-vinylpyrrolidone) (PVPON), (average Mw 1 300 000 g mol−1), tannic acid (TA), (Mw 1700 g mol−1), poly(ethylene imine) (PEI), (average Mw 25 000 g mol−1), and poly(methacrylic acid) (PMAA, Mw 21,800 g mol−1, Ð=1.32), mono- and dibasic sodium phosphate, glutaric aldehyde (GA), were purchased from Sigma-Aldrich. Poly(N-vinylcaprolactam) (PVCL), (Mw 20 800 g mol−1, Ð=1.19) was synthesized as described elsewhere.[lxix] Ultrapure (Siemens) filtered water with a resistivity of 18.2 MΩ cm was used for preparation of buffered solutions. Silica microparticles of 4 µm in diameter were purchased from Polysciences Inc. Chicken OVA323–339 peptide sequence (ISQAVHAAHAEINEAGR) and the BDC-2.5 mimotope (EKAHRPIWARMDAKK) were synthesized by Sigma Genosys. Western blot antibodies to STAT4 (C-20) and β-actin were purchased from Santa Cruz and Sigma, respectively. Anti-mouse IgG and anti-rabbit IgG secondary antibodies conjugated to horseradish peroxidase were purchased from Jackson Immunoresearch.
Mice
OT-II and NOD.BDC-2.5 TCR transgenic mice were bred and housed under specific pathogen-free conditions at the Research Support Building animal facility at the University of Alabama-Birmingham. Female mice at 8 weeks of age were used in all experiments. All animal studies were performed in accordance with the UAB Institutional Animal Use and Care Committee in compliance with the laws of the United States of America.
Preparation of TA-containing capsules
Hollow hydrogen-bonded shells (capsules) were prepared by coating 4.0±0.1 µm silica particles with (TA/PVPON)n or (TA/PVCL)n multilayer film followed by particle dissolution in 8% aqueous solution of hydrofluoric acid as established previously.[xxx] Specifically, 1.5 mL of 10%-aqueous suspension of particle were exposed to PVPON (1 mg mL−1) or to PVCL (0.5 mg mL−1) solutions in 0.01 M sodium phosphate at pH=3.5 for 10 min. After that, the silica particle suspension was pelleted in a 1.5 mL Eppendorf centrifuge tube and washed two times with 0.01 M sodium phosphate at pH=3.5 to remove unbound excess of polymers. Then, TA was allowed to adsorb onto particle surfaces from 0.5 mg mL−1 solution for 10 min. After each deposited layer, particles were centrifuged for 2 min at 2000 rpm and washed two times with the rinsing solution. Alternating coating of particles with the polymers was continued until the desired number of layers was achieved. Multilayer capsules were obtained by dissolving silica cores in aqueous hydrofluoric acid (8% wt) followed by their dialysis in de-ionized water for three days in the dark.[xxx] Capsules were vortexed and sonicated (15 s each) for 3 times before use in any experiments. Concentration of the capsule suspensions, [shells/microliter], was measured by capsule counting under an optical microscope using a hemocytometer.
Preparation of TA-free (PVPON)4 or (PVCL)4 capsules
The TA-free capsules were prepared as described in our previous work.[lxx,lxxi] Hydrogen-bonded multilayers of poly(methacrylic acid) with amino-containing poly(N-vinylpyrrolidone) (PVPON-NH2-7, Mw = 143884 g mol−1, Ð = 1.55) or poly(N-vinylcaprolactam) (PVCL-NH2−5, 23800, Ð = 1.5 ) copolymers with a certain molar percentage of amino group-containing polymer units were deposited on the silica particles. Assembly of the hydrogen-bonded layers was performed at pH=3.5, starting from PVPON-NH2 or PVCL-NH2 copolymer followed by PMAA. Each deposition cycle was followed by rinsing three times with a buffer solution at pH=3.5 to remove excess polymer, followed by centrifugation of suspensions at 2000 rpm for 2 min to remove supernatant. After four bilayers of (PVPON-NH2/PMAA) or (PVCL-NH2/PMAA) were deposited, chemical cross-linking of PVPON-NH2 or PVCL-NH2 layers was performed. For that, the core-shell particles were exposed to glutaric aldehyde solution (5 wt%) at pH=5 for (PVPON-NH2/PMAA)4 or pH=6.5 for (PVCL-NH2/PMAA)4 for 12 hours. After that, coated core-shell particles were exposed to pH=8.5 for 4 hours, followed by rinsing at pH=4. The core dissolution was performed as described above and resultant (PVPON)4 or (PVCL)4 capsules were purified by dialysis in de-ionized water for 3 days.
Primary Recall Assays and Cytokine Measurements by ELISA
NOD.BDC-2.5 or OT-II splenocyte single cell suspensions (5×105 cells) were seeded in a 96-well flat bottom plate and stimulated with 0.1 µM or 1 µM BDC-2.5 mimotope or 1 µM OVA323–339, respectively, in the presence or absence of 108–103 (PVPON/TA)4 shells in 200 µL total volume of Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum, 10 mM HEPES buffer, 4 mM L-glutamine, 2× non-essential amino acids, 1 mM sodium pyruvate, 61.5 µM 2-mercaptoethanol, and 100 µg mL−1 Gentamicin (Invitrogen) (complete DMEM). After incubation at 37°C in a 5% CO2 humid air chamber for 2, 3, or 4 days, supernatants were collected to examine cytokine synthesis. IFN-γ, IL-10, and IL-2 production was measured using antibody pairs from BD Biosciences as described previously.[lx] ELISA plates were read on a BioTek Synergy2 microplate reader (BioTek) and analyzed using Gen5 v.1.10 software (BioTek). MTT assay to assess cell viability was performed according to the manufacturer’s protocol (Sigma-Aldrich).
Oxidation of luminol to detect ROS synthesis
Luminol is cell permeable and readily oxidized by superoxide and hydrogen peroxide resulting in chemiluminescence and can be used to detect ROS synthesis with OT-II splenocytes as previously described.[xxv] Briefly, OT-II single cell suspensions were plated onto a 96-well round bottom plate at 5×105 cells in the presence or absence of 2.5×106 polymer shells in phenol red-free HBSS with 200 µM luminol (Sigma) and 0.32 Units mL−1 of horseradish peroxidase (Sigma) in a total volume of 200 µL. Luminescence was quantified using a SpectraMax L Luminescence microplate reader and was recorded every 2 minutes for 1 hour after stimulation with 100 ng mL−1 PMA and 1µg mL−1 ionomycin (In).
Western immunoblotting
Whole cell lysates were prepared as described previously,[lxxii] separated on a 4–20% gradient SDS-PAGE gel, and transferred onto 0.45 µm charged PVDF membranes. The membranes were incubated overnight at 4°C with antibodies against STAT4 or β-actin and exposed to the appropriate secondary antibody conjugated to horseradish peroxidase (Jackson ImmunoResearch). Chemiluminescence was detected with ECL plus (Amersham Pharmacia) and analyzed with Image Lab software v5.1 (Bio-Rad) to generate densitometry data.
Nitrite assay
NO2− production was measured by adding 50 µL of Griess reagent (1% sulphanilamide and 0.1% naphthylethylenediamide in 5% phosphoric acid) to 50 µL of tissue culture supernatant from primary recall assays. The NO2− concentration was determined by comparing the optical density at 550 nm with a standard curve generated from various known concentrations of sodium nitrite dissolved in culture medium.
Statistical Analysis
Data were analyzed using GraphPad Prism Version 5.0 statistical software. Determination of the difference between mean values for each experimental group was assessed using the 2-tailed Student’s t test, with p < 0.05 considered significant. All experiments were performed at least three separate times with data obtained in triplicate wells in each experiment.
Acknowledgments
This work was supported by NSF-DMR 1306110 (EK), ADA-7-12-CD-11 (HMT), and NIH NIAID 5T32AI007051-35 Immunologic Diseases and Basic Immunology T32 training grant (LEP). The following core facility was used to generate data for the manuscript: Animal Resources Program (G20RR025858, Sam Cartner, D.V.M., Ph.D.).
References
- i.Haskins K, Bradley B, Powers K, Fadok V, Flores S, Ling X, Pugazhenthi S, Reusch J, Kench J. Ann. NY Acad. Sci. 2003;1005:1043. doi: 10.1196/annals.1288.006. [DOI] [PubMed] [Google Scholar]
- ii.Lightfoot YL, Chen J, Mathews CE. Methods Mol. Biol. 2012;900:347. doi: 10.1007/978-1-60761-720-4_17. [DOI] [PubMed] [Google Scholar]
- iii.Padgett LE, Broniowska KA, Hansen PA, Corbett JA, Tse HM. Ann. NY Acad. Sci. 2013;1281:16. doi: 10.1111/j.1749-6632.2012.06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- iv.Tse HM, Thayer TC, Steele C, Cuda CM, Morel L, Piganelli JD, Mathews CE. J. Immunol. 2010;185:5247. doi: 10.4049/jimmunol.1001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- v.Varanasi V, Avanesyan L, Schumann DM, Chervonsky AV. Diabetes. 2012;61:2862. doi: 10.2337/db11-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vi.(a) Arif S, Moore F, Marks K, Bouckenooghe T, Dayan CM, Planas R, Vives-Pi M, Powrie J, Tree T, Marchetti P, Huang GC, Gurzov EN, Pujol-Borrell R, Eizirik DL, Peakman M. Diabetes. 2011;60:2112. doi: 10.2337/db10-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Honkanen J, Nieminen JK, Gao R, Luopajarvi K, Salo HM, Ilonen J, Knip M, Otonkoski T, Vaarala O. J. Immunol. 2011;185:1959. doi: 10.4049/jimmunol.1000788. [DOI] [PubMed] [Google Scholar]
- vii.Nicoletti F, Zaccone P, Di Marco R, Di Mauro M, Magro G, Grasso S, Mughini L, Meroni P, Garotta G. Endocrinology. 1996;137:5567. doi: 10.1210/endo.137.12.8940385. [DOI] [PubMed] [Google Scholar]
- viii.Trembleau S, Penna G, Bosi E, Mortara A, Gately MK, Adorini L. J. Exp. Med. 1995;181:817. doi: 10.1084/jem.181.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ix.Thayer TC, Delano M, Liu C, Chen J, Padgett LE, Tse HM, Annamali M, Piganelli JD, Moldawer LL, Mathews CE. Diabetes. 2011;60:2144. doi: 10.2337/db10-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- x.Hutchings P, Rosen H, O’Reilly L, Simpson E, Gordon S, Cooke A. Nature. 1990;348:639. doi: 10.1038/348639a0. [DOI] [PubMed] [Google Scholar]
- xi.El-Sheikh A, Suarez-Pinzon WL, Power RF, Rabinovitch A. J. Autoimmun. 1999;12:109. doi: 10.1006/jaut.1998.0264. [DOI] [PubMed] [Google Scholar]
- xii.Mandrup-Poulsen T, Pickersgill L, Donath MY. Nat. Rev. Endocrinol. 2010;6:158. doi: 10.1038/nrendo.2009.271. [DOI] [PubMed] [Google Scholar]
- xiii.Culina S, Brezar V, Mallone R. Eur. J. Endocrinol. 2013;168:R19. doi: 10.1530/EJE-12-0693. [DOI] [PubMed] [Google Scholar]
- xiv.Misler S. Islets. 2010;2:210. doi: 10.4161/isl.2.4.12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xv.(a) Froud T, Baidal DA, Ponte G, Ferreira JV, Ricordi C, Alejandro R. Cell Transplant. 2006;15:613. doi: 10.3727/000000006783981639. [DOI] [PubMed] [Google Scholar]; (b) Chatenoud L. N. Engl. J. Med. 2008;358:1192. doi: 10.1056/NEJMcibr0708067. [DOI] [PubMed] [Google Scholar]
- xvi.de Vos P, van Hoogmoed CG, van Zanten J, Netter S, Strubbe JH, Busscher HJ. Biomaterials. 2003;24:305. doi: 10.1016/s0142-9612(02)00319-8. [DOI] [PubMed] [Google Scholar]
- xvii.Panza JL, Wagner WR, Rilo HL, Rao RH, Beckman EJ, Russell AJ. Biomaterials. 2000;21:1155. doi: 10.1016/s0142-9612(99)00283-5. [DOI] [PubMed] [Google Scholar]
- xviii.Chandy T, Mooradian DL, Rao GH. Artificial organs. 1999;23:894. doi: 10.1046/j.1525-1594.1999.06244.x. [DOI] [PubMed] [Google Scholar]
- xix.Gattás-Asfura KM, Stabler CL. ACS Appl. Mater. Interfaces. 2013;5:9964. doi: 10.1021/am401981g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xx.Wilson JT, Cui W, Kozlovskaya V, Kharlampieva E, Pan D, Qu Z, Krishnamurthy VR, Mets J, Kumar V, Wen J, Song Y, Tsukruk V, Chaikof EL. J. Am. Chem. Soc. 2011;133:7054. doi: 10.1021/ja110926s. [DOI] [PubMed] [Google Scholar]
- xxi.Wilson JT, Chaikof EL. Adv. Drug Delivery Rev. 2008;60:124. doi: 10.1016/j.addr.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxii.Teramura Y, Iwata H. Transplantation. 2009;88:624. doi: 10.1097/TP.0b013e3181b230ac. [DOI] [PubMed] [Google Scholar]
- xxiii.Kizilel S, Scavone A, Liu X, Nothias JM, Ostrega D, Witkowski P, Millis M. Tissue Eng. A. 2010;16:2217. doi: 10.1089/ten.TEA.2009.0640. [DOI] [PubMed] [Google Scholar]
- xxiv.Tomei AA, Manzoli V, Fraker CA, Giraldo J, Velluto D, Najjar M, Pileggi A, Molano RD, Ricordi C, Stabler CL, Hubbell JA. Proc. Natl. Acad. Sci. U.S.A. 2014;111:10514. doi: 10.1073/pnas.1402216111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxv.Kozlovskaya V, Zavgorodnya O, Chen Y, Ellis K, Tse HM, Cui W, Thompson JA, Kharlampieva E. Adv. Funct. Mater. 2012;22:3389. doi: 10.1002/adfm.201200138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxvi.Shutava T, Prouty M, Kommireddy D, Lvov Y. Macromolecules. 2005;38:2850. [Google Scholar]
- xxvii.Erel-Unal I, Sukhishvili S. Macromolecules. 2008;41:3962. [Google Scholar]
- xxviii.Wilson JT, Cui W, Kozlovskaya V, Kharlampieva E, Pan D, Qu Z, Krishnamurthy VR, Mets J, Kumar V, Wen J, Song Y, Tsukruk V, Chaikof EL. J. Am. Chem. Soc. 2011;133:7054. doi: 10.1021/ja110926s. [DOI] [PubMed] [Google Scholar]
- xxix.Kozlovskaya V, Harbaugh S, Drachuk I, Shchepelina O, Kelley-Loughnane N, Stone M, Tsukruk VV. Soft Matter. 2011;7:2364. [Google Scholar]
- xxx.Chen J, Kozlovskaya V, Goins A, Campos-Gomez J, Saeed M, Kharlampieva E. Biomacromolecules. 2013;14:3830. doi: 10.1021/bm4008666. [DOI] [PubMed] [Google Scholar]
- xxxi.Park JH, Yang SH, Lee J, Ko EH, Hong D, Choi IS. Adv. Mater. 2014;26:2001. doi: 10.1002/adma.201304568. [DOI] [PubMed] [Google Scholar]
- xxxii.Zhuk I, Jariwala F, Attygalle AB, Wu Y, Libera MR, Sukhishvili SA. ACS Nano. 2014;8:7733. doi: 10.1021/nn500674g. [DOI] [PubMed] [Google Scholar]
- xxxiii.Ejima H, Richardson JJ, Liang K, Best JP, van Koeverden MP, Such GK, Cui J, Caruso F. Science. 2013;341:154. doi: 10.1126/science.1237265. [DOI] [PubMed] [Google Scholar]
- xxxiv.Shukla A, Fang JC, Puranam S, Jensen FR, Hammond PT. Adv. Mater. 2012;24:492. doi: 10.1002/adma.201103794. [DOI] [PubMed] [Google Scholar]
- xxxv.Rahim MA, Ejima H, Cho KL, Kempe K, Muellner M, Best JP, Caruso F. Chem. Mater. 2014;26:1645. [Google Scholar]
- xxxvi.Shutava TG, Balkundi SS, Vangala O, Steffan JJ, Bigelow RL, Cardelli JA, O’Neal DP, Lvov YM. ACS Nano. 2009;3:1877. doi: 10.1021/nn900451a. [DOI] [PubMed] [Google Scholar]
- xxxvii.Riedl KM, Hagerman AE. J. Agric. and Food. Chem. 2001;49:4917. doi: 10.1021/jf010683h. [DOI] [PubMed] [Google Scholar]
- xxxviii.Lopes GKB, Schulman HM, Hermes-Lima M. Biochim. Biophys. Acta. 1999;1472:142. doi: 10.1016/s0304-4165(99)00117-8. [DOI] [PubMed] [Google Scholar]
- xxxix.Hushulian DM, Fechina VA, Kazakov SV, Sakharov IY, Gazaryan IG. Biochemistry. 2003;68:1006. doi: 10.1023/a:1026016730127. [DOI] [PubMed] [Google Scholar]
- xl.Takebayashi J, Tai A, Yamamoto I. Biol. Pharm. Bull. 2003;26:1368. doi: 10.1248/bpb.26.1368. [DOI] [PubMed] [Google Scholar]
- xli.Shutova TG, Agabekov VE, Lvov YM. Russ. J. Gen. Chem. 2007;77:1494. [Google Scholar]
- xlii.Cao Y, He W. Acta Biomater. 2013;9:4558. doi: 10.1016/j.actbio.2012.08.037. [DOI] [PubMed] [Google Scholar]
- xliii.Lybaert L, De Vlieghere E, De Rycke R, Vanparijs N, De Wever O, De Koker S, De Geest BG. Adv. Funct. Mater. 2014 [Google Scholar]
- xliv.Dierendonck M, Fierens K, De Rycke R, Lybaert L, Maji S, Zhang Z, Zhang Q, Hoogenboom R, Lambrecht BN, Grooten J, Remon JP, De Koker S, De Geest BG. Adv. Funct. Mater. 2014;24:4634. [Google Scholar]
- xlv.Bottino R, Balamurugan AN, Tse H, Thirunavukkarasu C, Ge X, Profozich J, Milton M, Ziegenfuss A, Trucco M, Piganelli JD. Diabetes. 2004;53:2559. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]
- xlvi.Rincon M, Flavell RA, Davis RA. Free Radic. Biol. Med. 2000;28:1328. doi: 10.1016/s0891-5849(00)00219-7. [DOI] [PubMed] [Google Scholar]
- xlvii.Bottino R, Balamurugan AN, Bertera S, Pietropaolo M, Trucco M, Piganelli JD. Diabetes. 2002;51:2561. doi: 10.2337/diabetes.51.8.2561. [DOI] [PubMed] [Google Scholar]
- xlviii.Batinic-Haberle I, Spasojevic I, Tse HM, Tovmasyan A, Rajic Z, St. Clair DK, Vujaskovic Z, Dewhirst MW, Piganelli JD. Amino Acids. 2010;1:95. doi: 10.1007/s00726-010-0603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xlix.Hume PS, Anseth KS. J. Biomed. Mater. Res. A. 2011;99A:29. doi: 10.1002/jbm.a.33160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- l.Zavgorodnya O, Kozlovskaya V, Liang X, Kothalawala N, Catledge S, Dass A, Kharlampieva E. Materials Research Express. 2014;1:035039. [Google Scholar]
- li.Huber K, Krötz-Fahning M, Hock B. Protoplasma. 2006;229:221. doi: 10.1007/s00709-006-0206-y. [DOI] [PubMed] [Google Scholar]
- lii.Liu F, Kozlovskaya V, Zavgorodnya O, Martinez-Lopez C, Catledge S, Kharlampieva E. Soft Matter. 2014;10:9237. doi: 10.1039/c4sm01813c. [DOI] [PubMed] [Google Scholar]
- liii.Southern C, Schulster D, Green IC. FEBS Lett. 1990;276:42. doi: 10.1016/0014-5793(90)80502-a. [DOI] [PubMed] [Google Scholar]
- liv.Shiva S. Redox Biology. 2013;1:40. doi: 10.1016/j.redox.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- lv.Kharlampieva E, Sukhishvili SA. J. Macromol. Sci., Polym.Rev. 2006;46:377. [Google Scholar]
- lvi.Kozlovskaya V, Kharlampieva E, Drachuk I, Cheng D, Tsukruk VV. Soft Matter. 2010;6:3596. [Google Scholar]
- lvii.Kharlampieva E, Sukhishvili SA. Langmuir. 2003;19:1235. [Google Scholar]
- lviii.Tse HM, Milton MJ, Piganelli JD. Free Radical Biology & Medicine. 2004;36:233. doi: 10.1016/j.freeradbiomed.2003.10.029. [DOI] [PubMed] [Google Scholar]
- lix.Sklavos MM, Bertera S, Tse HM, Bottino R, He J, Beilke JN, Coulombe MG, Gill RG, Crapo JD, Trucco M, Piganelli JD. Diabetes. 2010;59:1731. doi: 10.2337/db09-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- lx.Tse HM, Milton MJ, Schreiner S, Profozich JL, Trucco M, Piganelli JD. J. Immunol. 2007;178:908. doi: 10.4049/jimmunol.178.2.908. [DOI] [PubMed] [Google Scholar]
- lxi.Howard M, O’Carra A. Immunol. Today. 1992;13:198. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- lxii.Bayer J, Gomer A, Demir Y, Amano H, Kish DD, Fairchild R, Heeger PS. Clin. Immunol. 2004;110:100. doi: 10.1016/j.clim.2003.10.006. [DOI] [PubMed] [Google Scholar]
- lxiii.Wu D, Wang J, Pae M, Meydani SN. Molecular Aspects of Medicine. 2012;33:107. doi: 10.1016/j.mam.2011.10.001. [DOI] [PubMed] [Google Scholar]
- lxiv.Jacobson NG, Szabo SJ, Weber-Nordt RM, Zhong Z, Schreiber RD, Darnell Jr JE, Murphy KM. J. Exp. Med. 1995;181:1755. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- lxv.Yamamoto K, Quelle FW, Thierfelder WE, Kreider BL, Gilbert DJ, Jenkins NA, Copeland NG, Silvennoinen O, Ihle JN. Mol. Cell. Biol. 1994;14:4342. doi: 10.1128/mcb.14.7.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- lxvi.Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC, Ihle JN. Nature. 1991;382:171. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- lxvii.(a) Muthian G, Bright JJ. J. Clin. Immunol. 2004;24:542. doi: 10.1023/B:JOCI.0000040925.55682.a5. [DOI] [PubMed] [Google Scholar]; (b) Wang J, Ren Z, Xu Y, Xiao S, Meydani SN, Wu D. The American Journal of Pathology. 2012;180:221. doi: 10.1016/j.ajpath.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- lxviii.Yu ES, Min HJ, An SY, Won HY, Hong JH, Hwang ES. Biochemical Pharmacology. 2008;76:70. doi: 10.1016/j.bcp.2008.03.020. [DOI] [PubMed] [Google Scholar]
- lxix.Liang X, Kozlovskaya V, Cox CP, Wang Y, Saeed M, Kharlampieva E. J. Polym. Sci. A: Polym. Chem. 2014 [Google Scholar]
- lxx.Liang X, Kozlovskaya V, Chen Y, Zavgorodnya O, Kharlampieva E. Chem. Mater. 2012;24:3707. doi: 10.1021/cm301657q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- lxxi.Kozlovskaya V, Wang Y, Higgins W, Chen J, Chen Y, Kharlampieva E. Soft Matter. 2012;8:9828. [Google Scholar]
- lxxii.Tse HM, Josephy SI, Chan ED, Fouts D, Cooper AM. J. Immunol. 2002;168:825. doi: 10.4049/jimmunol.168.2.825. [DOI] [PubMed] [Google Scholar]