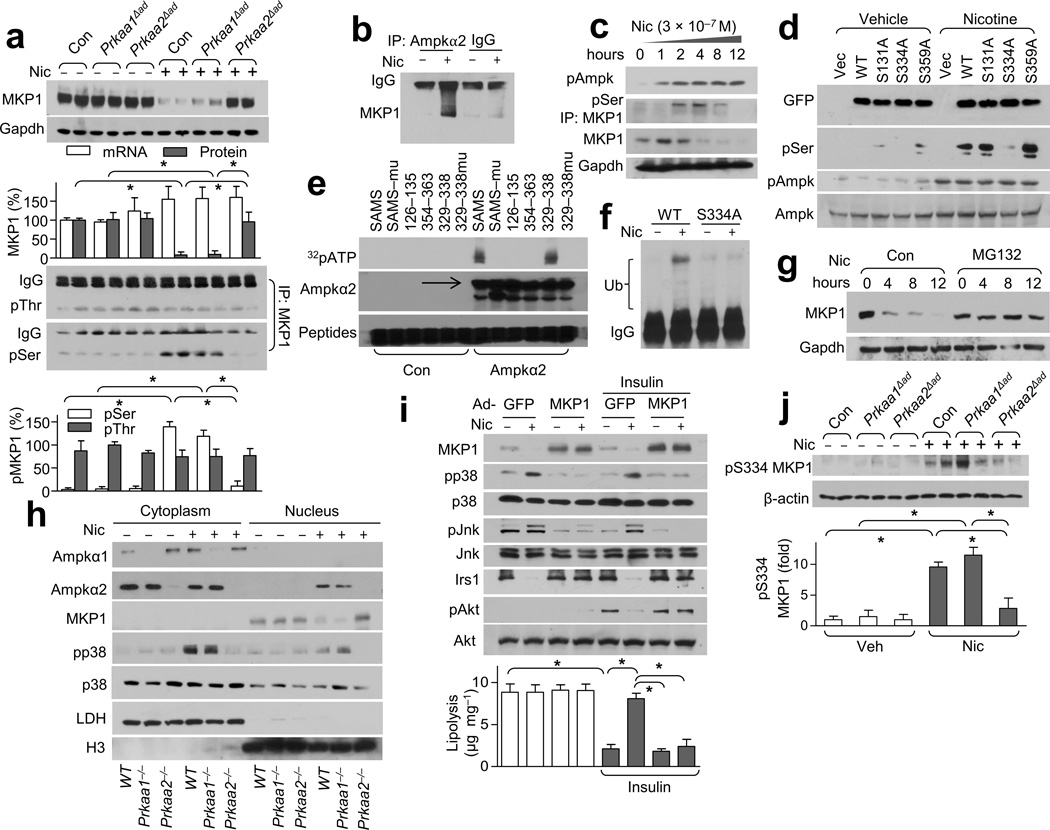
Figure 5.
Nicotine treatment results in greater pMKP1-Ser334 levels and subsequent degradation through AMPK. (a) MKP1 expression (top) and its serine (Ser) /threonine (Thr) phosphorylation (bottom) in WAT from Veh- or Nic-treated mice. Gapdh was detected as a loading control; n = 8 each. (b) AMPKα2-MKP1binding in MEF-derived adipocytes; n = 5 each. (c) AMPK activation and MKP1 serine phosphorylation in nicotine-treated isolated adipocytes; n = 4 each. (d) MKP1 phosphorylation in HEK293 cells transfected with vector, GFP-tagged WT MKP1 (WT) or the indicated site-directed mutants of MKP1; n = 4 each. (e) The indicated peptides (the SAMS peptide and its mutant, SAMSmu) or peptides spanning the indicated amino acid residues of MKP1; n = 3 each. (f) MKP1 ubiquitination in HEK293 cells transfected with WT MKP1 (WT) or MKP1-S334A; n = 4 each. (g) MKP1 protein expression in MG132-treated isolated adipocytes; n = 5 each. (h) The subcellular localization of AMPKα1/2, p38 and MKP1 in adipocytes treated with or without nicotine. LDH (lactate dehydrogenase), cytoplasmic marker; H3 (histone 3), nuclear marker; n = 5 each. (i) Insulin signaling (western blot, top) and lipolysis (bottom) in Nic- or Veh-treated MEF-derived adipocytes transfected with adenovirus GFP (Ad-GFP) or MKP1 (Ad-MKP1).; n = 5 each. (j) Detection of pMKP1-S334 in WAT from the indicated strains in Nic- or Veh-treated mice (top) and its quantitation (bottom). Beta-actin was detected as a loading control; n = 6 each. Significance determined by one-way ANOVA with Bonferroni’s post-hoc test (a,i,j) and *P < 0.05. All values are means ± SEM.