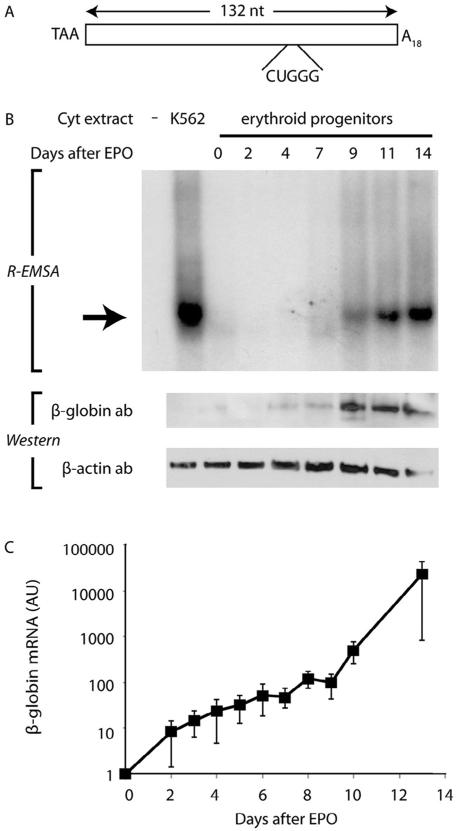
Fig. 1.
Assembly of the β-complex and coordinate induction of β-globin expression during erythropoiesis. (A) Schematic depiction of the 132-nt in vitro transcribed [32P]-labeled RNA, corresponding to the full-length β-globin 3′UTR, that is used for all EMSAs. The translation termination codon (TAA), 18-nt poly(A) tail (A18), and 5-nt β-complex target site (CUGGG) are indicated. (B) β-Globin protein accumulates in primary erythroid cells that support β-complex formation. Top: R-EMSAs were conducted on a [32P]-labeled β-globin RNA 3′UTR using cytoplasmic extracts purified from human EPO-induced CD34+ cells at defined intervals. K562 cytoplasmic extract was used as a positive control. The β-complex is indicated by an arrow. Bottom: Parallel Western-transfer analyses of cytoplasmic extracts for β-globin and control β-actin expression. (C) β-Complex assembly in erythroid differentiation parallels an exponential increase in β-globin mRNA. RT-qPCR analyses were conducted on RNA isolated from human EPO-induced CD34+ erythroid progenitor cells. Each point represents the mean±SEM values from at least 4 biological replicates, normalized to values for endogenous control β-actin and GAPDH mRNAs. The normalized level of β-globin mRNA at day 0 was arbitrarily assigned unit value. Note logarithmic ordinate scale.