Fig. 6.
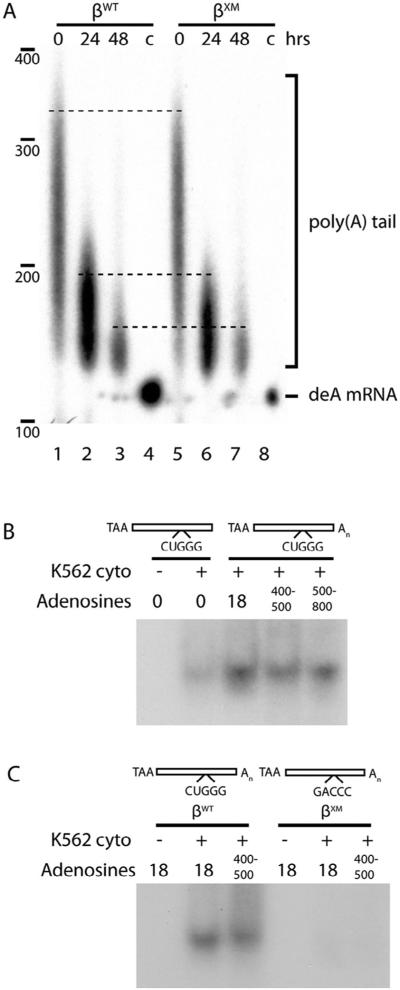
The β-complex promotes binding of PABPC1 to the proximal region of the poly(A) tail. (A) Abolition of the β-complex does not materially affect the length of the β-globin mRNA poly(A) tail. Stably expressed βWT and βXM mRNAs were recovered from K562 cells at defined intervals following transcriptional arrest with tetracycline, and poly(A) tail lengths determined by ligase-mediated RT-PCR. The migration of deadenylated β-globin mRNA (deA mRNA; lanes 4, 8) was used as negative control. (B) The efficiency of β-complex assembly requires a minimal poly(A) tail, but is not enhanced by additional polyadenylate sequence. R-EMSAs were conducted on a [32P]-labeled β-globin 3′UTR RNAs containing poly(A) tails of varying lengths (Sup Fig. S4A), using cytoplasmic extracts from K562 cells. (C) The β-complex does not assemble on βXM RNA that contains an elongated poly(A) tail. As in panel B, using [ 32P]-labeled βWT and βXM 3′UTR RNAs containing variably-lengthed poly(A) tails (Sup Fig. S4B).