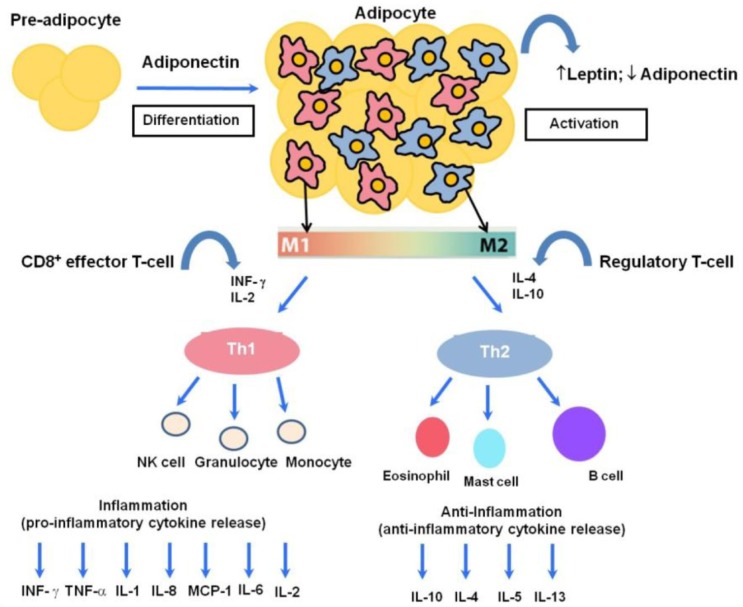
Fig. 1.
The human host immune response to a variety of inflammatory states involves a balance between pro-inflammatory and anti-inflammatory cytokines. Adiponectin can act in an autocrine fashion and leads to proliferation, differentiation and activation from preadipocytes to adipocytes. One hallmark of obesity is inflamed adipose tissue leading to macrophage recruitment. Activated adipose tissue is responsible for the secretion of leptin and adiponectin, while increased numbers of macrophages cause phenotypic and morphological changes. These changes include the polarization of adipose tissue macrophages toward a classical M1 or alternatively-activated M2 phenotype, which are associated with potent pro-inflammatory and anti-inflammatory activities, respectively. M1 arises in response to IFN-γ and IL-2, while M2 macrophages arise in response to various immunomodulatory factors such as IL-4, IL-10 and IL-13. In low-grade chronic inflammation associated with obesity, there is a phenotypic shift from M2 to M1, with a prominent M1 macrophage infiltration, consequently augmenting the release of T helper type-1 cytokines such as IL-1, IL-2, IL-6, TNF, monocyte chemo-attractant protein (MCP)-1 and interferon gamma (IFN-γ). Adipocytes can directly and indirectly interact with a wide variety of circulating immunocytes such as granulocytes, NK cells, and monocytes as part of the pro-inflammatory response; and eosinophils, mast cells and B cells as part of the anti-inflammatory response, eventually leading to aberrant immune homeostasis.