Figure 2.
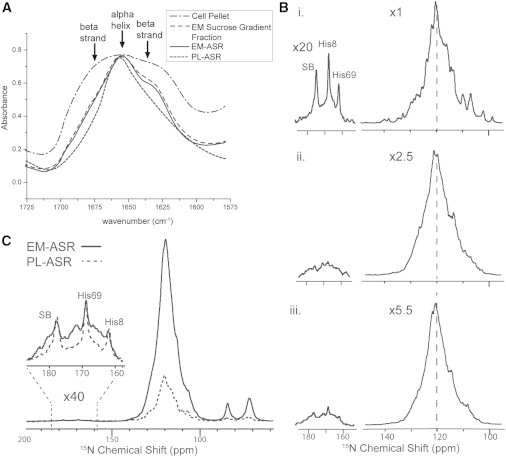
(A) FTIR spectra of NA EM-ASR throughout the inner membrane isolation process. By observing the narrowing of the Amide I peak and the reduction of its β-shoulder, the reduction of outer membrane content (β-barrel proteins) can be monitored. (B) One-dimensional 15N spectra of (i) UCN PL-ASR, (ii) UN EM-ASR, and (iii) rbUN EM-ASR. The relative increase in UN-labeled α-helical proteins in rbUN EM-ASR is evident from the relative increase in the downshifted maximum of the spectra (dashed line). All spectra are scaled to approximately match intensities, with scaling factors, and corrected for the number of scans taken in each experiment (noted to the left). The main peak is presented with no window function, whereas 60 Hz of line broadening is applied to the regions of the three resolved peaks. (C) One-dimensional 15N spectra of UCN PL-ASR and rbUN EM-ASR with the entire spectra processed with the stronger exponential window function of 60 Hz, and scaled such that the three resolved peaks (SB, His8, and His69) approximately match in intensity, showing that ASR accounts for only ∼1/4 of the labeled protein content in EM-ASR.
