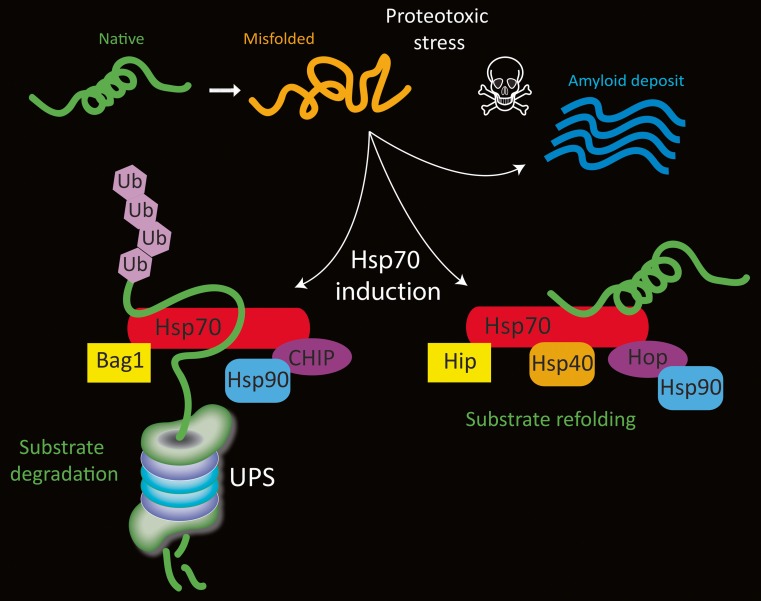
Fig. 1.
Schematic of Hsp70 (red) and a few of its cofactors. Under physiological conditions, misfolded proteins can induce Hsp70 gene expression and they may be degraded by the ubiquitin proteasome system (UPS) if they are irreparably damaged. During substrate degradation, CHIP binds the C-terminal of Hsp70 and acts as an E3 ubiquitin ligase to mark the protein for removal. During this process, Bag1 binds the ATPase domain at the N-terminal and serves to link the Hsp70/cofactor complex with the UPS. On the other hand, when proteins can be refolded to their native structure and salvaged, Bag-1 binding is blocked by the cochaperone Hip and CHIP binding is blocked by Hop. Hsp40 and Hsp90 may also bind the protein refolding complex and promote ATP-dependent folding activity. Not shown in this figure is the dimerization of some of these proteins. Under conditions of severe proteotoxic stress or loss of homeostasis, proteins may not undergo degradation or refolding and are sequestered in fibrillar deposits, such as Lewy bodies in Parkinson’s disease