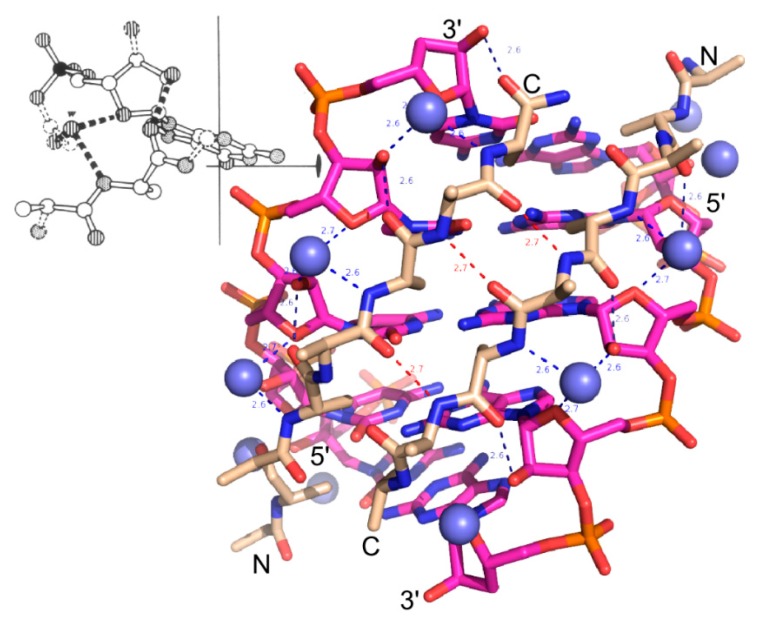
Figure 1.
Stereochemistry of peptide-RNA conformational complementarity [1]. The minor groove in double-stranded RNA (magenta) complements the preferred right-hand twist of antiparallel β hairpin structures (wheat). Adjacent nucleotide and peptide strands are parallel (5'-3'; N-C) and the two sets are antiparallel. Van der Waals distances between peptide and nucleotide components are optimal precisely at a peptide radius for which there are exactly two amino acids per base. (Inset) The double-double helix is also stabilized by recurring hydrogen bonds between peptide carbonyl and the ribose 2'OH groups and between amide nitrogens and water molecules (blue spheres) between the ribose O1 and 2'OH groups. The resulting hydrogen-bonded network stabilizes a ribose orientation such that the 3'OH group is poised to serve as a nucleophile for polymerization.