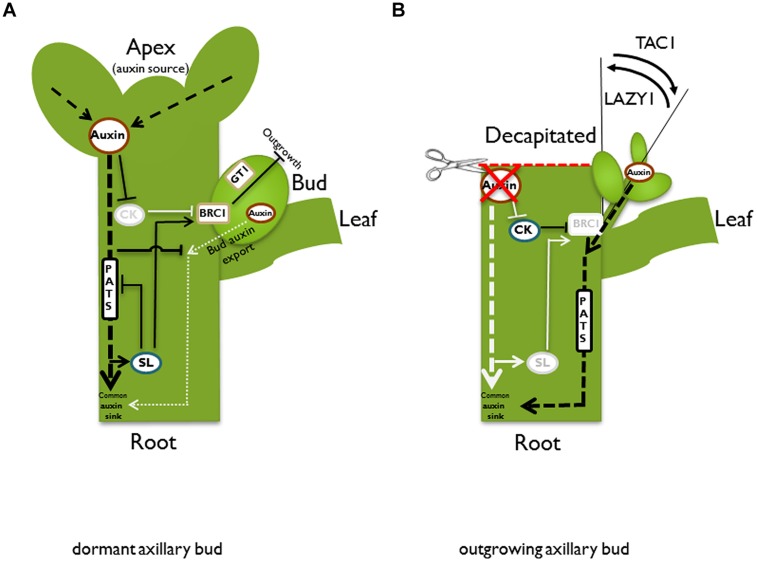
FIGURE 2.
Schematic illustration of different pathways and models in the control of bud outgrowth. In an intact plant (A), the apex is a strong auxin source. Auxin is transported basipetally in the polar auxin transport stream (PATS). According to the second messenger model, auxin promotes strigolactone (SL) and represses cytokinin (CK) biosynthesis, respectively. Both hormones have adverse effects on bud outgrowth, most likely acting via the transcription factor BRANCHED1/TEOSINTE BRANCHED1 (BRC1/TB1). Auxin indirectly promotes BRC1/TB1 expression, which suppresses bud outgrowth. GRASSY TILLERS1 (GT1) is a putative downstream target for TB1 in monocots. According to the auxin transport canalization model, the axillary bud is also an auxin source and as a prerequisite for vascular tissue formation and bud outgrowth, it has to establish its own auxin export. However, it competes with the shoot apex for the stem as a shared auxin sink. This competition is enhanced by SL, which reduces plasma membrane accumulation of the PIN1 auxin efflux carrier and therefore inhibits the PATS in the main stem. High auxin levels in the stem prevent the formation of an initial auxin export flux from the bud, and therefore suppress bud outgrowth. After decapitation (B), the apex as the primary auxin source is removed. Biosynthesis of SL is not promoted anymore, while repression of CK biosynthesis is released. Furthermore, the auxin level in the main stem is reduced and thus the sink capacity is increased, facilitating the establishment of an initial auxin export from the bud. After bud outgrowth, the emanating branch takes over the function of the lost apex as the primary auxin source and re-establishes apical dominance. Both described models, the second messenger model and the auxin transport canalization model, are not mutually exclusive, and the described pathways could contribute to bud outgrowth control simultaneously. After bud outgrowth, the angle of the branch is also under control. TILLER ANGLE CONTROL1 (TAC1) increases the tiller angle in monocots, while LAZY1 has the opposite function and reduces the tiller angle. Black lines and letters designate active pathways; light gray lines and letters indicate suppression or down-regulation of the respective pathway.