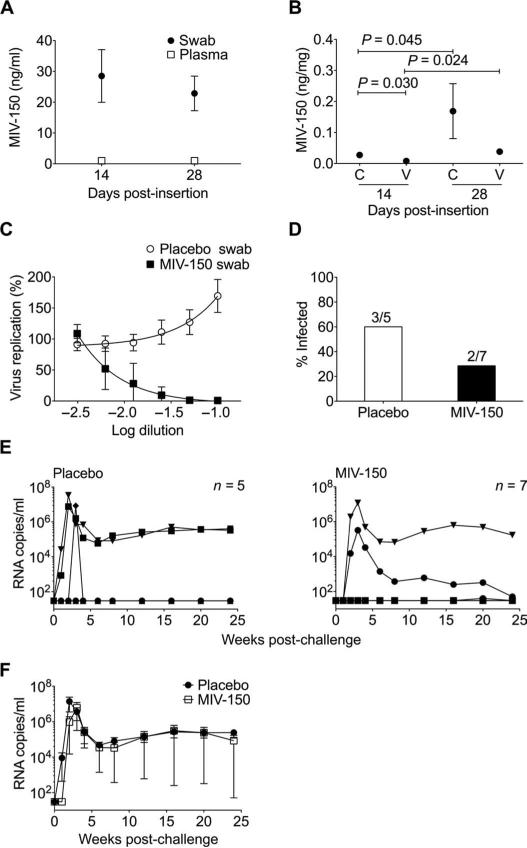
Fig. 3.
MIV-150 silicone IVRs offer partial protection against SHIV-RT infection. (A and B) MIV-150 in plasma and vaginal swabs 14 days (n = 13) and 28 days (n = 12) after insertion (A) and in cervical (C) and vaginal (V) pinch biopsies at 14 days (n = 6) and 28 days (n = 6) after insertion (B). Mean ± SEM is shown in both (A) and (B). Statistical analyses in (A) and (B) used the Mann-Whitney U test. (C) Percent of in vitro HIV replication in the presence of vaginal swab fluids from animals that received MIV-150 (n = 6) and placebo IVRs (n = 10; mean ± SEM) (relative to no swab controls, set as 100%). (D) Infection frequency in the placebo and MIV-150 IVR groups. The numbers above each bar denote the number of infected animals over the number of challenged animals in each group. (E) Viremia (SIV RNA copies per milliliter of plasma) shown over time for each animal challenged with 103 TCID50 SHIV-RT 2 weeks after receiving placebo or MIV-150 IVRs. (F) Mean ± SEM plasma viral load of infected animals from each group.