Figure 3.
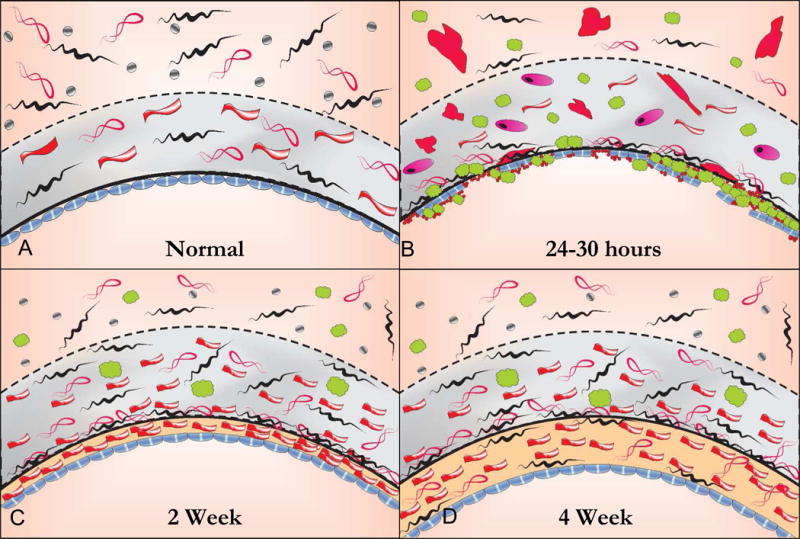
The histology of the healing autogenous vein graft. In the normal vein, the intima is lined by large flat endothelial cells that are more permeable than those in arteries. The intima is separated from the media by a fenestrated internal elastic laminae. The tunica media is thin compared to an artery with 2 or more layers of smooth muscle cells (SMC) while the adventitia is relatively thick consisting of a loose collagenous network interspersed with fibroblasts and vaso vasorum and small autonomic nerves, A. Within 24 hours following implantation the vein grafts exhibit significant endothelial cell loss and sub-endothelial edema. Inflammatory cells, platelets and fibrin are seen adherent to the surface and infiltrating underneath the attenuated endothelial cell monolayer. There is edema in the tunica media with extensive SMC necrosis or swelling and hypertrophy of the remaining SMCs with infiltration of inflammatory cells, B. By 2 to 4 weeks there is re-endothelialization of the luminal surface and a developing neointima. While the endothelium is continuous, it remains dysfunctional as evidenced by organelle hypertrophy and adhesion molecule expression. The medial edema and inflammation is reduced and there is increased collagen content. Surviving medial SMCs appear hypertrophic with increased rough endoplasmic reticulum and Golgi apparatus indicating synthetic transformation. Over time the adventitia becomes incorporated in surrounding tissue and vaso vasorum and adrenergic nerve fibers grow in from adjacent arteries and connective tissue, C. By 4 weeks there is a predominant layer of intimal thickening characterized by SMCs embedded in a matrix of collagen and ground substance. While early medial thickening is caused predominantly by edema and inflammation, fibrous transformation is responsible for late medial thickening. Areas of the medial wall are devoid of cells and entirely replaced by fibrosis. Clinically stiffening of the vein graft is likely from from the increase in fibrous protein as well as increased cross linking of extracellular matrix proteins.
