Abstract
Purpose
This study aims to investigate Saccharomyces boulardii CNCM I-745 during Helicobacter pylori eradication in children.
Methods
One hundred ninety-four H. pylori positive children were randomized in two groups. Therapy (omeprazole+clarithromycin+amoxicillin or omeprazole+clarithromycin+metronidazole in case of penicillin allergy) was given to both groups during two weeks. In the treatment group (n: 102) S. boulardii was added to the triple therapy, while the control group (n: 92) only received triple therapy. The incidence, onset, duration and severity of diarrhea and compliance to the eradication treatment were compared. A 13C urea breath test was done 4 weeks after the end of eradication therapy in two groups of 21 patients aged 12 years and older to test the H. pylori eradication rate.
Results
In the treatment group, diarrhea occurred in 12 cases (11.76%), starting after 6.25±1.24 days, lasting 3.17±1.08 days, and compliance to eradication treatment was 100%. In the control group, diarrhea occurred in 26 cases (28.26%), starting after 4.05±1.11 days, lasting 4.02±0.87 days, and in six cases eradication treatment was stopped prematurely (p<0.05). The 13C urea breath test showed successful H. pylori eradication in 71.4% of the patients in the treatment and in 61.9 % in the control group (not significant).
Conclusion
S. boulardii has a beneficial effect on the prevention and treatment of diarrhea during H. pylori eradication in children. Although S. boulardii did only slightly increase H. pylori eradication rate, compliance to eradication treatment was improved.
Keywords: Child, Compliance, Helicobacter pylori, Diarrhea, Prevention & control, Probiotics, Yeasts
INTRODUCTION
Epidemiological data suggest that up to 50% to 80% of the children in developing countries are Helicobacter pylori carriers [1]. Standardized eradication treatment of H. pylori requires a combination of antibiotics, usually administered during one to two weeks. Therefore, diarrhea develops frequently during H. pylori eradication. This diarrhea not only increases the morbidity, but also increases the economic burden of the treatment. Saccharomyces boulardii CNCM I-745 has been shown to be an effective probiotic in several types of acute diarrhea. In this study, we aimed to demonstrate the efficacy of S. boulardii in the prevention of diarrhea during H. pylori eradication treatment in children.
MATERIALS AND METHODS
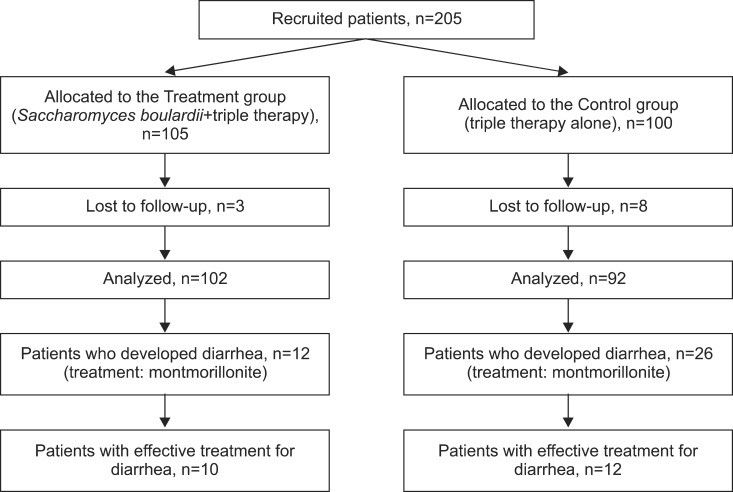
Children presenting with chronic abdominal pain or chronic vomiting were investigated for organic disease. Two hundred and five children in whom H. pylori infection was diagnosed were included in this study (Fig. 1). Since 11 patients were lost in follow-up, data of 194 patients, between 22 months and 16 years old were collected. All patients were diagnosed with H. pylori infection through serological quantification of H. pylori immunoglobulin G (IgG) antibodies (122 cases) or histological evaluation (72 cases). In a subgroup of 42 children between 12 and 16 years old (mean age of 13.33±1.26 years) a 13C urea breath test (UBT) was done four weeks after the end of the eradication therapy to confirm eradication.
Fig. 1. Flow diagram of patients.
All patients were given oral triple eradication therapy during two weeks (omeprazole+amoxicillin+clarithromycin, omeprazole+metronidazole+clarithromycin for participants with a penicillin allergy). The treatment group received two sachets of S. boulardii orally per day, starting on the first day of the antibiotic therapy (250 mg per sachet; Biocodex, Paris, France, Chinese brand name: YiHuo) during two weeks. If diarrhea occurred, patients in either group received montmorillonite powder (3 g three times daily, Smecta; Beaufour Ipsen Pharmaceuticals, Paris, France) orally without interrupting the S. boulardii treatment.
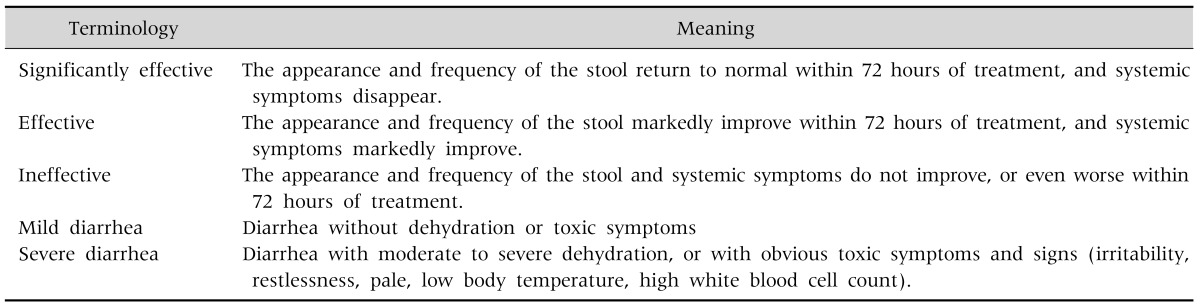
The primary outcome was the incidence of diarrhea defined as the percentage of patients developing a diarrhea. Diarrhea was defined as an increase in the frequency of bowel movements (>3/day) or decrease in stool consistency (Bristol stool scale 5 or 6). Secondary outcomes were: i) the onset of diarrhea, which was defined as the time between the inclusion of the patient and the onset of diarrhea; ii) the duration of diarrhea, which was defined as the number of days with diarrhea (the end of the diarrhea was defined as the moment when consistency (Bristol stool scale <4) or frequency (<3 stools/day) of the stools returned to normal); iii) the eradication rate; iv) compliance, defined as the number of patients who completed the entire course of the triple eradication therapy; v) efficacy of the diarrhea treatment; vi) severity of diarrhea [2] (Table 1). The efficacy of the treatment was defined as the percentage of patients in whom the eradication treatment resulted in a negative 13C UBT.
Table 1. Efficacy of Treatment and Severity of Diarrhea.
The sample size was calculated using the following formula N=Z2×[p×(1-P)]/E2=174 where N is the sample size, Z the confidence interval (=1.96), E the sampling error (=0.05) and P the incidence of AAD (=0.13). Data analysis was performed using SPSS 11.5 statistical software (Softonic; Softonic International S.A., http://en.softonic.com/s/spss-11.5-statistical-software-free-download). The onset time and duration of diarrhea were analyzed using two-tailed t-tests; p<0.05 was considered statistically significant. This study received approval from ethics committee of Daping Hospital of The Third Military Medical University (No. 2003009).
RESULTS
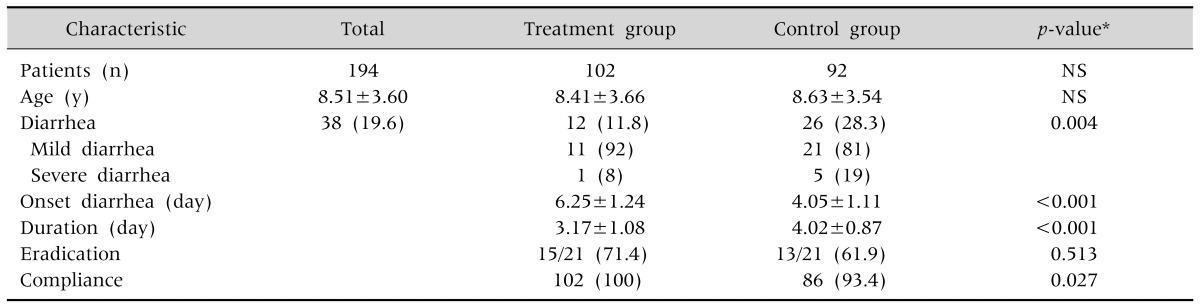
Data of 194 (90 male, 104 female) patients were analyzed. In the treatment group, 12/102 (11.8%) patients developed diarrhea, while in the control group 26/92 (28.3%) patients had diarrhea (p<0.05) (Table 2). The onset of diarrhea in the treatment group was 6.25±1.24 days, what was later than in the control group (4.05±1.11 days; p<0.001). The duration of diarrhea in the treatment group was 3.17±1.08 days, which was significantly shorter than in the control group (4.02±0.87 days; p<0.001) (Table 2).
Table 2. Patient Characteristics.
Values are presented as number only, mean±standard deviation, or number (%).
NS: not significant.
*By two-tailed t-test.
H. pylori was eradicated in the treatment group in 15/21 cases (eradication rate: 71.4%) and in 13/21 cases (eradication rate: 61.9%) in the control group. The difference in H. pylori eradication rate was not statistically significant (p=0.513, Table 2). No patient discontinued H. pylori treatment prematurely in the treatment group while six patients in the control group stopped H. pylori treatment without medical advice, resulting in a statistically significant difference (p=0.027, Table 2). In the treatment group, we observed 11 cases of mild diarrhea and 1 case of severe diarrhea. In the control group, there were 26 cases with diarrhea, of whom 21 were mild and 5 severe (p<0.05, Table 2).
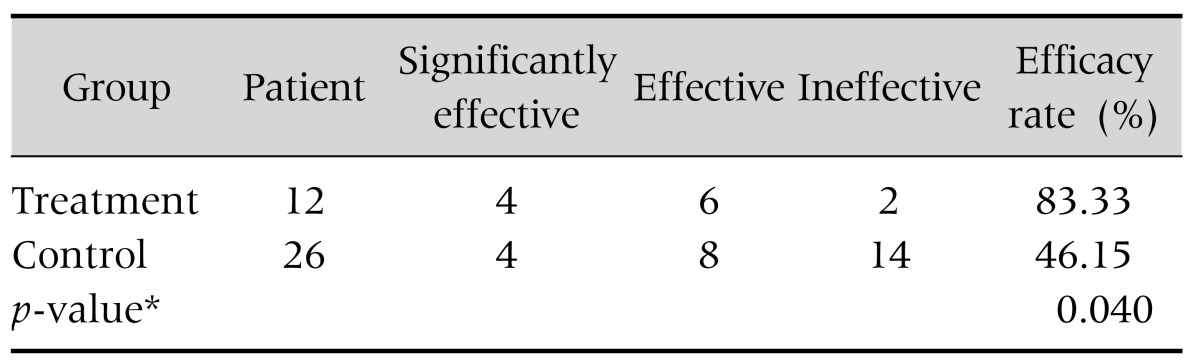
In the treatment group, montmorillonite powder was effective in 10/12 (83.3%) cases that developed diarrhea. In the control group, montmorillonite powder was effective in only 46.1% (p<0.05, Table 3).
Table 3. Comparison of Treatment Efficacy for Diarrhea (Montmorillonite).
*By Fisher's exact test.
DISCUSSION
The normal microbiome contributes to digestion, stimulates the immune system and provides a mechanical barrier called "colonization resistance" [3,4]. This involves the interaction of many bacterial microorganisms and results in a barrier effect against colonization by pathogenic organisms. Factors such as fasting, surgery and antibiotics disrupt this protective barrier. H. pylori eradication treatment includes proton pump inhibitor and two antibiotics out of three: amoxicillin, clarithromycin and metronidazole [5]. Proton pump inhibitors increase intragastric pH levels, what contributes to dysbiosis [6]. Due to these adverse effects on the intestinal micro-ecosystem environment, patients receiving H. pylori eradication treatment are prone to a series of gastrointestinal disorders such as bloating, anorexia, diarrhea and constipation [7]. One study reported an incidence of 30.9% of antibiotic associated diarrhea in children during H. pylori eradication [8]. According to a meta-analysis, the incidence of diarrhea during H. pylori eradication is 12.2% [9]. Our study evidenced that S. boulardii is an effective option to prevent diarrhea during H. pylori eradication treatment in children. This effect is observed for the incidence, delay in onset and duration of the diarrhea. The results of this study indicate that treatment with S. boulardii has the potential to significantly shorten the duration of diarrhea [10,11]. Antibiotics result in host susceptibility to pathogen colonization until the normal microbiome has recovered. Probiotics are uniquely qualified to fit into this window of susceptibility and may act as surrogate normal microflora until recovery is achieved [12].
Studies reported that the mechanisms of action of S. boulardii in the treatment of diarrhea could include the following 4 aspects: i) effects of anti-bacterial toxins; ii) direct or indirect inhibition of growth of intestinal pathogens, such as Candida albicans, Salmonella, Yersinia; iii) increase of short chain fatty acids concentrations; iv) immunomodulatory effects such as an increase in sIgA [13]. H. pylori interacts with the host immune response by upregulation of various cytokines, such as tumor necrosis factor-α, interferon-γ, interleukin (IL)-1, IL-6 and IL-8 [14]. These cytokines interact through a complex inflammatory immune regulatory network of paracrine and endocrine pathways and act on B lymphocytes, natural killer cells, macrophages [14]. This results in a specific and non-specific immune response [15,16]. The level of IL-8 is positively correlated with the severity of inflammation [17]. Studies in a mouse ileum loop model showed that S. boulardii inhibits the activation of ERK1/2 and MAPkinase which inhibit IL-8 production, and limits the activation of T-helper lymphocytes in order to inhibit the inflammatory response [18,19]. S. boulardii inhibits the inflammatory signaling pathways, by inhibiting the translocation of NF-κB in inflammatory signaling pathways, exhibiting anti-inflammatory properties [20,21].
There has been widespread controversy whether probiotics can be used in the treatment of H. pylori infection. Gotteland et al. [22] demonstrated that S. boulardii increases the eradication rate with 12%, which is similar to our finding. Szajewska et al. [9] reported in a meta-analysis that S. boulardii given along with triple therapy significantly increased the eradication rate and reduced the overall risk of H. pylori therapy-related adverse effects, particularly of diarrhea. It is not clear if the higher eradication rate is due to a specific probiotic activity on H. pylori, by improving the efficacy of the eradication treatment or by improving compliance.
In our study, diarrhea was treated with montmorillonite powder, a natural clay [23]. The major mode of action is as an absorbans of water [23]. A meta-analysis showed that this product is effective and safe in the treatment of diarrhea [23]. The efficacy of the product is also acknowledged in the recommendations for the treatment of acute gastroenteritis by the European Society of Paediatric Gastroenterology, Hepatology and Nutrition [24]. In our study, montmorillonite was found to be more effective in the treatment group that received concomitant S. boulardii. This may be related to the fact that the diarrhea in this group started later and was less severe. S. boulardii contributes to the decrease of diarrhea by providing a restoration of the gastro-intestinal flora.
Our study does have some limitations as it is an open study. The diagnosis of H. pylori was not according to standard techniques since serology is not accepted as a reliable diagnostic tool in children [5]. However, the proportion of patients diagnosed by serology in the treatment group and control group did not show any statistical difference. The proportion of patients diagnosed by serology in the subgroups of those who developed diarrhea and those who had conducted UBTs also did not show any statistical difference. Therefore, although bias regarding the inclusion of false positive patients in our study may have possibly existed, bias regarding allocation among groups was resolved. Moreover, this study focusses on the decrease of adverse effects linked to H. pylori eradication and not on H. pylori pathology and diagnostic techniques.
In conclusion, the yeast probiotic did prevent diarrhea associated with H. pylori standard eradication treatment with a proton pump inhibitor and two antibiotics. Also, when diarrhea developed, it was less severe and of shorter duration in the S. boulardii group. S. boulardii increased the compliance to H. pylori eradication therapy which may be related to a small increase of H. pylori eradication with 10 percent. However, about 65% of the children were diagnosed on the basis of serology. A comparable percentage of those developing diarrhea were diagnosed on serology, as was the case in those in whom a UBT was performed. The findings of this trial need to be confirmed with a prospective double blind study in which diagnosis is based on histology and culture.
ACKNOWLEDGEMENTS
Yvan Vandenplas is a consultant for United Pharmaceuticals and Biocodex.
References
- 1.Jafri W, Yakoob J, Abid S, Siddiqui S, Awan S, Nizami SQ. Helicobacter pylori infection in children: population-based age-specific prevalence and risk factors in a developing country. Acta Paediatr. 2010;99:279–282. doi: 10.1111/j.1651-2227.2009.01542.x. [DOI] [PubMed] [Google Scholar]
- 2.Yang XQ, Yi ZW. Pediatrics. 6th ed. Beijing: People's Medical Publishing House; 2003. pp. 295–296. [Google Scholar]
- 3.Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis. 2009;15:300–310. doi: 10.1002/ibd.20602. [DOI] [PubMed] [Google Scholar]
- 4.Preidis GA, Versalovic J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology. 2009;136:2015–2031. doi: 10.1053/j.gastro.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koletzko S, Jones NL, Goodman KJ, Gold B, Rowland M, Cadranel S, et al. H pylori Working Groups of ESPGHAN and NASPGHAN. Evidence-based guidelines from ESPGHAN and NASPGHAN for Helicobacter pylori infection in children. J Pediatr Gastroenterol Nutr. 2011;53:230–243. doi: 10.1097/MPG.0b013e3182227e90. [DOI] [PubMed] [Google Scholar]
- 6.Wallace JL, Syer S, Denou E, de Palma G, Vong L, McKnight W, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314–1322. doi: 10.1053/j.gastro.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi K, Maekawa T, Nakagawa K, Chouno S, Hayakumo T, Tomono N, et al. Efficacy and safety of Helicobacter pylori eradication therapy with omeprazole, amoxicillin and high- and low-dose clarithromycin in Japanese patients: a randomised, double-blind, multicentre study. Clin Drug Investig. 2006;26:403–414. doi: 10.2165/00044011-200626070-00002. [DOI] [PubMed] [Google Scholar]
- 8.Hurduc V, Plesca D, Dragomir D, Sajin M, Vandenplas Y. A randomized, open trial evaluating the effect of Saccharomyces boulardii on the eradication rate of Helicobacter pylori infection in children. Acta Paediatr. 2009;98:127–131. doi: 10.1111/j.1651-2227.2008.00977.x. [DOI] [PubMed] [Google Scholar]
- 9.Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069–1079. doi: 10.1111/j.1365-2036.2010.04457.x. [DOI] [PubMed] [Google Scholar]
- 10.Swidsinski A, Loening-Baucke V, Verstraelen H, Osowska S, Doerffel Y. Biostructure of fecal microbiota in healthy subjects and patients with chronic idiopathic diarrhea. Gastroenterology. 2008;135:568–579. doi: 10.1053/j.gastro.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Barc MC, Charrin-Sarnel C, Rochet V, Bourlioux F, Sandré C, Boureau H, et al. Molecular analysis of the digestive microbiota in a gnotobiotic mouse model during antibiotic treatment: influence of Saccharomyces boulardii. Anaerobe. 2008;14:229–233. doi: 10.1016/j.anaerobe.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 12.McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. 2010;16:2202–2222. doi: 10.3748/wjg.v16.i18.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandenplas Y, Brunser O, Szajewska H. Saccharomyces boulardii in childhood. Eur J Pediatr. 2009;168:253–265. doi: 10.1007/s00431-008-0879-7. [DOI] [PubMed] [Google Scholar]
- 14.Cadamuro AC, Rossi AF, Maniezzo NM, Silva AE. Helicobacter pylori infection: host immune response, implications on gene expression and microRNAs. World J Gastroenterol. 2014;20:1424–1437. doi: 10.3748/wjg.v20.i6.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beswick EJ, Suarez G, Reyes VE. H. pylori and host interactions that influence pathogenesis. World J Gastroenterol. 2006;12:5599–5605. doi: 10.3748/wjg.v12.i35.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdel-Latif MM, Windle H, Terres A, Eidhin DN, Kelleher D, Reynolds JV. Helicobacter pylori extract induces nuclear factor-kappa B, activator protein-1, and cyclooxygenase-2 in esophageal epithelial cells. J Gastrointest Surg. 2006;10:551–562. doi: 10.1016/j.gassur.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Lopes AI, Quiding-Jarbrink M, Palha A, Ruivo J, Monteiro L, Oleastro M, et al. Cytokine expression in pediatric Helicobacter pylori infection. Clin Diagn Lab Immunol. 2005;12:994–1002. doi: 10.1128/CDLI.12.8.994-1002.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Kokkotou EG, Mustafa N, Bhaskar KR, Sougioultzis S, O'Brien M, et al. Saccharomyces boulardii inhibits ERK1/2 mitogen-activated protein kinase activation both in vitro and in vivo and protects against Clostridium difficile toxin A-induced enteritis. J Biol Chem. 2006;281:24449–24454. doi: 10.1074/jbc.M605200200. [DOI] [PubMed] [Google Scholar]
- 19.Dalmasso G, Cottrez F, Imbert V, Lagadec P, Peyron JF, Rampal P, et al. Saccharomyces boulardii inhibits inflammatory bowel disease by trapping T cells in mesenteric lymph nodes. Gastroenterology. 2006;131:812–825. doi: 10.1053/j.gastro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Thomas S, Przesdzing I, Metzke D, Schmitz J, Radbruch A, Baumgart DC. Saccharomyces boulardii inhibits lipopolysaccharide-induced activation of human dendritic cells and T cell proliferation. Clin Exp Immunol. 2009;156:78–87. doi: 10.1111/j.1365-2249.2009.03878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fidan I, Kalkanci A, Yesilyurt E, Yalcin B, Erdal B, Kustimur S, et al. Effects of Saccharomyces boulardii on cytokine secretion from intraepithelial lymphocytes infected by Escherichia coli and Candida albicans. Mycoses. 2009;52:29–34. doi: 10.1111/j.1439-0507.2008.01545.x. [DOI] [PubMed] [Google Scholar]
- 22.Gotteland M, Poliak L, Cruchet S, Brunser O. Effect of regular ingestion of Saccharomyces boulardii plus inulin or Lactobacillus acidophilus LB in children colonized by Helicobacter pylori. Acta Paediatr. 2005;94:747–751. doi: 10.1111/j.1651-2227.2005.tb01848.x. [DOI] [PubMed] [Google Scholar]
- 23.Dupont C, Vernisse B. Anti-diarrheal effects of diosmectite in the treatment of acute diarrhea in children: a review. Paediatr Drugs. 2009;11:89–99. doi: 10.2165/00148581-200911020-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guarino A, Ashkenazi S, Gendrel D, Lo Vecchio A, Shamir R, Szajewska H. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidencebased guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59:132–152. doi: 10.1097/MPG.0000000000000375. [DOI] [PubMed] [Google Scholar]