Abstract
Therapy with trastuzumab confers a survival benefit in HER2 positive advanced gastric and gastroesophageal adenocarcinoma. HER2 status is evaluated by immunohistochemistry (IHC) and in situ hybridization (ISH). An ISH ratio of HER2 to centromere 17 (CEP17) ≥2.0 is considered amplified. This assumes that CEP17 reflects chromosomal copy number. Cases where CEP17 exceeds 3 are classified as polysomic, but it's unknown if they represent true polysomy or centromeric amplification. This has implications on the validity of current ISH criteria. Multiplex ligation-dependent probe amplification (MLPA) allows simultaneous quantification of multiple loci and can distinguish between true polysomy and centromeric amplification. We selected 13 gastric cancers with CEP17 counts ≥3.0 (polyCEP17), and 8 non-polyCEP17 gastric cancer controls. Silver ISH for HER2 and CEP17 were performed and scored by manufacturer guidelines. We also performed an MLPA HER2 assay that evaluates 22 genes on chromosome 17. MLPA identified HER2 amplification in 7 polyCEP17 cases compared to 2 identified by ISH. Overall, 9 of 13 polyCEP17 cases had amplification of the peri-centromeric gene WSB1, compared to 1 of 8 non-polyCEP17 controls (p=0.02). This could account for ISH CEP17 counts ≥3.0. MLPA did not show any cases of complete chromosome 17 duplication and peri-centromeric amplification can explain most cases of ISH polyCEP17. Current ISH criteria may under-diagnose HER2 amplification in polyCEP17 cases due to flawed assumptions about polysomy. MLPA can detect HER2 amplification missed by IHC and ISH, and thus may be an effective ancillary technique in evaluating HER2 status.
Keywords: gastric cancer, HER2, polysomy, multiplex ligation-dependent probe amplification, in situ hybridization
Introduction
Gastric and gastroesophageal junction (GEJ) adenocarcinoma is an aggressive disease with a 5-year survival rate of 5-20% in advanced disease 1. The international Trastuzumab for Gastric Cancer (ToGA) trial demonstrated that patients with human epidermal receptor 2 (HER2) overexpressing tumours benefit from targeted therapy with trastuzumab 1. This has resulted in the routine evaluation of HER2 status in gastric and GEJ adenocarcinomas by immunohistochemistry (IHC) and in situ hybridization (ISH) 1-3.
Currently for ISH, a HER2 to chromosome 17 centromere (CEP17) ratio ≥ 2.0 or an average HER2 count > 6.0 can be considered positive 1-3. However, the ISH CEP17 count sometimes averages higher than 2, the expected value for diploid cells. Some of this is due to cell overlap or non-specific probe binding. However, there are cases where the counts are noticeably and consistently higher than expected. Cases where CEP17 counts are above 3 are usually designated as "chromosome 17 polysomic". Approximately 4% of the patients enrolled in the ToGA trial fit into this category 4.
Several possibilities exist for the ISH "polysomy" observation. Firstly some of the cells could simply be actively replicating its DNA, which would lead to increased copies of most of its DNA. The other explanations include either true chromosomal duplication(s) or localized amplification of the centromeric region that complements the CEP17 probe 5. The use of ratios in HER2 ISH assumes that CEP17 accurately represents chromosome 17 copy number 2,3,5. It is assumed that when CEP17 and HER2 are proportionately increased, HER2 is not truly amplified. However, this assumption fails if localized centromeric amplification and not true polysomy 17 is the reason for the increased CEP17 count. In breast cancer, it has been demonstrated that true polysomy 17 is relatively rare compared to localized centromeric amplification 5,6. This has not been investigated in gastric and GEJ cancer, but it has implications for current diagnostic criteria and eligibility for targeted therapy.
While HER2 ISH involves only 2 probes, multiplex methods are able to provide additional information on other loci. Multiplex ligation-dependent probe amplification (MLPA) is a multiplex polymerase chain reaction (PCR) technique that employs a large number of probes targeting multiple genes 5,7-9. The PCR portion of this process only requires two primers which specifically amplify hybridized and enzymatically ligated probes. MLPA allows us to explore whether gastric cancers with higher than normal CEP17 counts (polyCEP17) represent true polysomy 17 or localized peri-centromeric amplification. The results can impact current ISH criteria.
Materials and Methods
Research ethics board approval was obtained from Sunnybrook Health Sciences Centre (REB #285-2010). Primary gastric and GEJ adenocarcinoma biopsies (223 cases in total) from Sunnybrook Health Science Centre between the years 2000 to 2011 were examined for HER2 status 10. Cases were stained and evaluated for HER2 IHC (Ventana, Tucson, AZ, USA) and silver ISH (Ventana) using gastric cancer criteria 1-3. Cases which demonstrated ISH CEP17 averaging ≥ 3.0 were defined as polyCEP17 3. CEP17 counts were also evaluated in 3 separate regions in these cases, with 20 consecutive tumour cells evaluated in each region. PolyCEP17 was defined as focal or multifocal depending on whether 1 or >1 regions demonstrated CEP17 counts ≥ 3.0.
Four non-polyCEP17 HER2 negative gastric cancer cases, four non-polyCEP17 HER2 positive cases, and two normal gastric tissue samples served as controls. Controls were randomly chosen from the known subgroups.
The tumour slides were evaluated and the cancer circled. Tumour regions were manually microdissected from additional unstained sections. DNA was isolated using RecoverAll total nucleic acid isolation kit (Life Technologies, Burlington, ON, Canada). DNA was subjected to MLPA with the SALSA ERBB2 (HER2) assay (MRC Holland, Amsterdam, Netherlands). Data was analyzed by Coffalyser.Net software (MRC Holland), with results standardized to the normal gastric controls. Genes with MLPA ratios < 0.7 were defined as copy number loss; ≥ 0.7 < 1.5 were considered normal; ≥ 1.5 were defined as low (“heterozygous”) amplified; ≥ 2.0 were defined as highly amplified 7,9. When multiple probes were used for a specific gene, the probe with the highest ratio (best affinity) was considered to be most representative. For statistical analysis, categorical data was compared with Fisher's exact test, and continuous data with Student's t-test.
Results
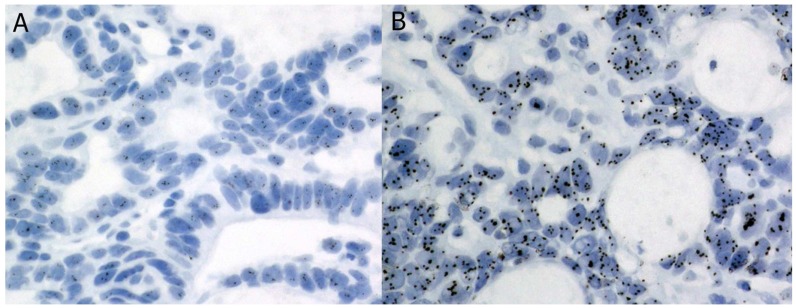
Of the gastric cancer biopsies we reviewed, 26 displayed polyCEP17 on ISH 10. Of these, 13 had sufficient remaining tumour tissue for further analysis. Our controls consisted of 8 gastric cancers without polyCEP17 and 2 normal gastric tissues. Their characteristics are summarized in Table 1. The ISH of a polysomic case is shown in Figure 1.
Table 1.
Clinical characteristics of polyCEP17 and non-polyCEP17 cases
| PolyCEP17 (n = 13) | Non-polyCEP17 (n = 8) | |
|---|---|---|
| Grade | ||
| Well | 1 | 0 |
| Moderate | 6 | 3 |
| Poor | 6 | 5 |
| Subtype | ||
| Intestinal | 10 | 4 |
| Diffuse | 1 | 1 |
| Mixed | 2 | 3 |
| IHC | ||
| 0 / 1+ | 4 | 3 |
| 2+ | 7 | 1 |
| 3+ | 2 | 4 |
| HER2 ISH ratio | ||
| < 2 | 11 | 4 |
| ≥ 2.0 | 2 | 4 |
| Mean CEP17 count | 3.56 | 2.04 |
| polyCEP17 focality | ||
| Focal | 6 | N/A |
| Multifocal | 7 | N/A |
Figure 1.
Silver in situ hybridization for a case of polyCEP17 with HER2 signals (a) and CEP17 signals (b).
MLPA amplification results for HER2 and peri-centromeric gene WSB1 are compared to ISH results in Table 2. Cases with multifocal versus focal polyCEP17 did not differ in their likelihood of WSB1 amplification by MLPA (p = 0.26). One case showed HER2 high amplification by MLPA with low amplification of WSB1; this case had a HER2 count of 5.45 by ISH and a ratio of 1.7, and thus was HER2 negative by current ISH guidelines. There were a further 4 cases that were MLPA low amplified for HER2. Of these cases, 3 demonstrated WSB1 amplification. Furthermore, these 3 cases all had HER2 ISH counts over 4 but had low ISH ratios due to polyCEP17. Table 3 examines the concordance between absolute ISH HER2 counts and MLPA ratios at various cutoffs.
Table 2.
MLPA versus ISH results
| MLPA amplification |
ISH | |||
|---|---|---|---|---|
| polyCEP17 (n = 13) |
Non-polyCEP17 (n = 8) |
|||
| HER2+ | HER2- | HER2+ | HER2- | |
| HER2 | ||||
| High | 2 | 1 | 2 | 0 |
| Low | 0 | 4 | 1 | 0 |
| Non-amplified | 0 | 6 | 1 | 4 |
| WSB1 | ||||
| High | 0 | 2 | 0 | 0 |
| Low | 1 | 6 | 0 | 1 |
| Non-amplified | 1 | 3 | 4 | 3 |
Table 3.
Concordance between HER2 ISH count and MLPA at different cutoffs
| ISH HER2 count cutoffs | ||||||||
|---|---|---|---|---|---|---|---|---|
| MLPA cutoffs | ≥ 4.0 | < 4.0 | ≥ 5.0 | < 5.0 | ≥ 6.0 | < 6.0 | ||
| ≥ 1.5 | 9 | 1 | 6 | 4 | 5 | 5 | ||
| < 1.5 | 3 | 8 | 1 | 10 | 0 | 11 | ||
| Concordance | 81% | 76% | 76% | |||||
| ≥ 1.8 | 7 | 0 | 6 | 1 | 5 | 2 | ||
| < 1.8 | 5 | 9 | 1 | 13 | 0 | 14 | ||
| Concordance | 76% | 90% | 90% | |||||
| ≥ 2.0 | 5 | 0 | 5 | 0 | 4 | 1 | ||
| < 2.0 | 7 | 9 | 2 | 14 | 1 | 15 | ||
| Concordance | 76% | 90% | 90% | |||||
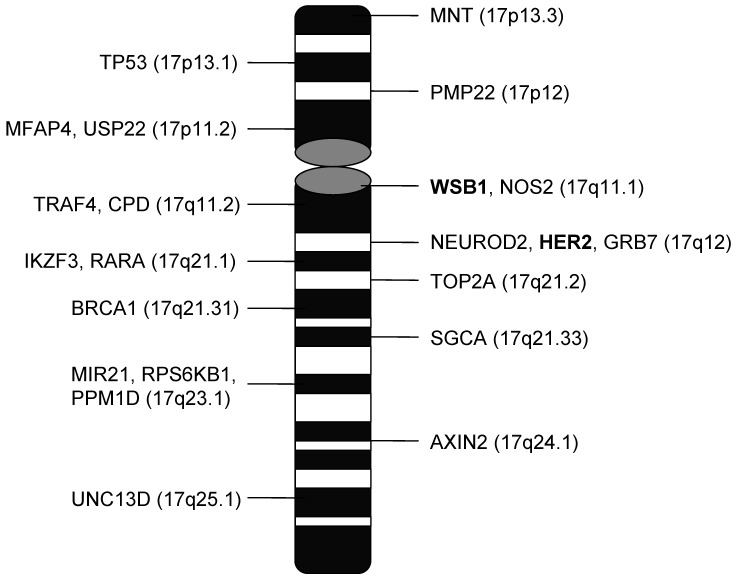
Table 4 and 5 summarize the amplification and loss data for all probed genes on chromosome 17. Figure 2 shows the location of these genes on chromosome 17. Only WSB1 and BRCA1 showed higher rates of copy number gain in the polyCEP17 group compared to the non-polyCEP17. There were no cases which showed amplification in all chromosome 17 genes. One case had 9 genes on 17q that were highly amplified, 7 of which were genes clustered around HER2. Cases with WSB1 amplification had more copy number alterations on other chromosome 17 genes compared to WSB1 non-amplified cases (p < 2.6 x 10-5).
Table 4.
Percentage of cases with MLPA amplification by gene
| Gene* | % Cases with MLPA amplification | P-value | |
|---|---|---|---|
| ISH polyCEP17 | Non- polyCEP17 | ||
| 17p | |||
| MNT | 0.0 | 12.5 | 0.38 |
| TP53 | 15.4 | 12.5 | 1.00 |
| 17q | |||
| WSB1 | 69.2 | 12.5 | 0.023 |
| TRAF4 | 23.1 | 37.5 | 0.63 |
| CDP | 38.5 | 12.5 | 0.34 |
| NEUROD2 | 46.2 | 25.0 | 0.40 |
| HER2 | 53.8 | 37.5 | 0.66 |
| GRB7 | 38.5 | 50.0 | 0.67 |
| IKZF3 | 61.5 | 25.0 | 0.18 |
| RARA | 7.7 | 25.0 | 0.53 |
| TOP2A | 69.2 | 25.0 | 0.081 |
| BRCA1 | 46.2 | 0.0 | 0.046 |
| MIR21 | 69.2 | 37.5 | 0.20 |
| RPS6KB1 | 15.4 | 0.0 | 0.53 |
| PPM1D | 7.7 | 0.0 | 1.00 |
*Genes PMP22, MFAP4, USP22 (17p); NOS2, SGCA, AXIN2, and UNC13D (17q) were not amplified in any samples.
Table 5.
Percentage of cases with MLPA copy number loss by gene
| Gene* | % Cases with MLPA amplification | P-value | |
|---|---|---|---|
| ISH polyCEP17 | Non- polyCEP17 | ||
| 17p | |||
| MNT | 15.4 | 0 | 0.50 |
| MFAP4 | 15.4 | 12.5 | 1.00 |
| USP22 | 7.7 | 12.5 | 1.00 |
| 17q | |||
| NOS2 | 7.7 | 0 | 1.00 |
| SGCA | 7.7 | 0 | 1.00 |
| AXIN2 | 15.4 | 0 | 0.50 |
| UNC13D | 7.7 | 12.5 | 1.00 |
*Genes not displayed did not show any loss by MLPA in any cases
Figure 2.
Loci of chromosome 17 genes assessed by MLPA.
Discussion
By MLPA, complete chromosome 17 duplication was not observed in our cohort of gastric and GEJ cancers, even in the presence of polyCEP17. Two tumours had gains in 9 of 17 genes on 17q with many consecutive probes showing gains. These could represent regional amplification of a portion of 17q. This suggests that significant portions of peri-centromeric and peri-HER2 chromosomal regions can be co-amplified in gastric cancer.
Most cases with polyCEP17 demonstrate amplification of the peri-centromeric gene WSB1 or WD repeat and SOCS box containing 1. This gene may be involved in Hedgehog signaling and DNA damage response 11,12. It is not specifically amplified in any carcinomas and its functions are poorly understood. Its location at 17q11.1 is adjacent to the centromeric region of chromosome 17, and its amplification is highly correlated with ISH polyCEP17 5.
In one polyCEP17 case, the patient showed HER2 high amplification by MLPA as well as low amplification of CEP17. In this instance, the polyCEP17 caused the ISH ratio to be less than 2. The average HER2 ISH count was less than 6, and this tumour was also 2+ equivocal on IHC. Thus this patient was HER2 negative by every standard criteria and not eligible for trastuzumab. There were another 4 polyCEP17 cases that showed low level HER2 amplification on MLPA (cutoff of 1.5). Three of these were WSB1 amplified and had average absolute HER2 counts above 4. If we used the average CEP17 count of non-polyCEP17 cases (2.04), all 3 would have been ratio positive. Our institutional experience based on previous review of over 200 primary gastric cancer biopsies also suggests that the average non-polyCEP17 case has a CEP17 count of 2.07 10. This means that between 8-31% of polyCEP17 cases in our dataset could have been misclassified by ISH. In our opinion, ancillary techniques such as MLPA may help to clarify HER2 status in polyCEP17 cases with average absolute ISH HER2 copy number >4 and ≤6 due to the possibility of misclassification on ISH.
Our MLPA data raises several issues with respect to cutoffs. Firstly, the above cases demonstrate that up to 31% of polyCEP17 cases with HER2 and WSB1 co-amplification could be falsely negative on ISH. We note that the cutoff ratio for amplification is different between MLPA and ISH (1.5 vs 2.0) and attribute this to underlying differences between the techniques. While ISH focuses only on a small region with the highest HER2 counts, MLPA examines a larger area of tissue with potential stromal contaminants. Thus the MLPA ratio will usually be lower than that of ISH. Unlike ISH, MLPA ratios are standardized to normal tissue and thus would not be affected by the CEP17 count. Previous studies in breast cancer using the 1.5 MLPA cutoff have demonstrated very high concordance with ISH 7,9. Array comparative genomic hybridization studies also use 1.5 as the cutoff ratio for amplification 13,14. Nonetheless, to address this issue we looked at various other possible MLPA and HER2 count cutoffs (table 3). An MLPA cut-off of 1.5 was best at identifying cases with HER2 counts ≥ 4. Based on our discovery that most polyCEP17 is due to pericentromeric amplification, such cases would be considered HER2 amplified if we assumed a true chromosome 17 count of 2. More stringent criteria revealed no differences in concordance when the MLPA cutoff was 1.8 or 2.0 with a HER2 absolute count cutoff of either 5 or 6. However, a HER2 absolute cutoff of 5 and MLPA cutoff of 1.8 classified more patients as HER2 positive, potentially qualifying them for therapy.
MLPA is a powerful tool to simultaneously investigate copy number alterations on multiple genes. The cases with WSB1 amplification had more copy number alterations on other chromosome 17 genes. Amongst the most frequently amplified included TOP2A and MIR21. TOP2A encodes the topoisomerase IIα enzyme and its amplification has been associated with resistance to anthracyclines 15,16. MIR21 has been shown to be upregulated in the majority of gastric cancers and its microRNA product, miR-21, may confer chemoresistance to cisplatin 17,18. While BRCA1 amplification was associated with polyCEP17, copy number gain of BRCA1 is not known to be associated with gastric cancer. It is possible that BRCA1 amplification is a result of its proximity to TOP2A, as BRCA1 amplification was only seen in the presence of TOP2A amplification. We also noticed that the GRB7 gene, located immediately downstream of HER2, is only amplified in the presence of HER2 amplification. Interestingly, GRB7 has been shown to be a HER2 dependent oncogene that enhances phosphorylation of HER2 19. Thus while some of the genetic aberrations may be occurring randomly or by physical proximity, the genes with frequent copy number gains often have known roles in promoting tumour progression or resistance.
There are advantages and limitations to consider in our study. MLPA can provide copy number information about a large panel of genes (up to 50) simultaneously using as little as 20ng of input DNA 5,7-9. While stromal contaminants can bias MLPA results, proper tissue block selection and manual microdissection is sufficient to overcome this 7. The fact that our polyCEP17 cases showed significantly more WSB1 amplification than non-polyCEP17 cases suggests an adequate degree of tumour enrichment through manual dissection. Furthermore, despite the concern that MLPA may miss focal phenomenon, there was no statistical difference in the ability of MLPA to pick up WSB1 amplification in focal versus multifocal polyCEP17 cases. The MLPA cutoffs of 1.5 and 2.0 for low and high amplification were established based on comparative data between MLPA and ISH in breast cancer 7,9. However, it is important to concede that MLPA has not been clinically validated in large cohorts of unselected gastric cancer. Given the ability of MLPA to clarify difficult ISH cases, we feel that this technique has the potential to be an effective ancillary technique pending such large scale validation.
While the sample size of our study was small, to our knowledge we are the first to specifically address the issue of polysomy 17 in gastric cancer in the context of HER2 status. Our results suggest that most cases of ISH "polysomy" are the result of peri-centromeric amplification and not whole chromosome 17 duplication. This raises concerns as to the validity of standard ISH ratios in polyCEP17 cases. Current guidelines could under-diagnose HER2 amplification in these circumstances and result in treatment ineligibility. An average absolute HER2 count cut-off may be more appropriate for polyCEP17 cases. However, there is still insufficient validation of the current absolute HER2 cutoff of >6 in gastric cancer, which is based primarily on expert opinion and experience from breast cancer 3,20. Thus in our opinion, the optimal cut-off in gastric cancer deserves further debate and investigation. Our study has also demonstrated the potential of MLPA as an ancillary technique to clarify polyCEP17 in gastric cancer in equivocal cases. We do not suggest replacing ISH with MLPA as each has their merits. Instead we propose that MLPA may be a complementary technique employed in cases that pose difficulty on ISH.
Acknowledgments
The authors wish to thank the staff at the Sunnybrook Research Institute's Genomics Core Facility for their assistance in this project. We wish to thank Roche Canada and the Canada Foundation for Innovation for supporting this research.
References
- 1.Bang YJ, Van Cutsem E, Feyereislova A. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann M, Stoss O, Shi D. et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 3.Ruschoff J, Hanna W, Bilous M. et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637–50. doi: 10.1038/modpathol.2011.198. [DOI] [PubMed] [Google Scholar]
- 4.Chung H, Bang YJ, Xu JM. et al. Human epidermal growth factor receptor 2 (HER2) in gastric cancer (GC): results of the ToGA trial screening programme and recommendations for HER2 testing. Eur J Cancer Suppl. 2009;7:364. [Google Scholar]
- 5.Moelans CB, de Weger RA, van Diest PJ. Absence of chromosome 17 polysomy in breast cancer: analysis by CEP17 chromogenic in situ hybridization and multiplex ligation-dependent probe amplification. Breast Cancer Res Treat. 2010;120:1–7. doi: 10.1007/s10549-009-0539-2. [DOI] [PubMed] [Google Scholar]
- 6.Tse CH, Hwang HC, Goldstein LC. et al. Determining true HER2 gene status in breast cancers with polysomy by using alternative chromosome 17 reference genes: implications for anti-HER2 targeted therapy. J Clin Oncol. 2011;29:4168–74. doi: 10.1200/JCO.2011.36.0107. [DOI] [PubMed] [Google Scholar]
- 7.Moelans CB, de Weger RA, Ezendam C, van Diest PJ. HER-2/neu amplification testing in breast cancer by multiplex ligation-dependent probe amplification: influence of manual- and laser microdissection. BMC Cancer. 2009;9:4. doi: 10.1186/1471-2407-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moelans CB, de Weger RA, Monsuur HN, Vijzelaar R, van Diest PJ. Molecular profiling of invasive breast cancer by multiplex ligation-dependent probe amplification-based copy number analysis of tumor suppressor and oncogenes. Mod Pathol. 2010;23:1029–39. doi: 10.1038/modpathol.2010.84. [DOI] [PubMed] [Google Scholar]
- 9.Moelans CB, de Weger RA, van Blokland MTM, van der Wall E, van Diest PJ. Simultaneous detection of TOP2A and HER2 gene amplification by multiplex ligation-dependent probe amplification in breast cancer. Mod Pathol. 2010;23:62–70. doi: 10.1038/modpathol.2009.136. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh E, Henry P, Kwok K, Hanna W. HER2/Neu testing in 207 gastric and gastroesophageal junction adenocarcinomas: immunohistochemistry and silver in situ hybridization (SISH) provide effective brightfield methods for clinical HER2 testing. Modern Pathology. 2012;25(Suppl 2):163A. [Google Scholar]
- 11.Dentice M, Bandyopadhyay A, Gereben B. et al. The Hedgehog-inducible ubiquitin ligase subunit WSB-1 modulates thyroid hormone activation and PTHrP secretion in the developing growth plate. Nat Cell Biol. 2005;7:698–705. doi: 10.1038/ncb1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong Y, Li QG, Xing TY, Zhang M, Zhang JJ, Xia Q. HIF1 regulates WSB-1 expression to promote hypoxia-induced chemoresistance in hepatocellular carcinoma cells. FEBS Lett. 2013;587:2530–5. doi: 10.1016/j.febslet.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Rossi E, Klersy C, Manca R, Zuffardi O, Solcia E. Correlation between genomic alterations assessed by array comparative genomic hybridization, prognostically informative histologic subtype, stage, and patient survival in gastric cancer. Human Pathol. 2011;42:1937–45. doi: 10.1016/j.humpath.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Yang S, Jeung HC, Jeong HJ, Choi YH, Kim JE, Jung JJ, Rha SY, Yang WI, Chung HC. Identification of genes with correlated patterns of variations in DNA copy number and gene expression level in gastric cancer. Genomics. 2007;89:451–9. doi: 10.1016/j.ygeno.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Kanta SY, Yamane T, Dobashi Y, Mitsui F, Kono K, Ooi A. Topoisomerase IIa gene amplification in gastric carcinomas: correlation with the HER2 gene. An immunohistochemical, immunoblotting, and multicolor fluorescence in situ hybridization study. Human Pathol. 2006;37:1333–43. doi: 10.1016/j.humpath.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Liang Z, Zeng X, Gao J. et al. Analysis of EGFR, HER2, and TOP2A gene status and chromosomal polysomy in gastric adenocarcinoma from Chinese patients. BMC Cancer. 2008;8:363. doi: 10.1186/1471-2407-8-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan SH, Wu CW, Li AF, Chi CW, Lin WC. miR-21 microRNA expression in human gastric carcinomas and its clinical association. Anticancer Res. 2008;28:907–11. [PubMed] [Google Scholar]
- 18.Yang S, Huang C, Li X, Yu M, He Y, Li J. miR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicol. 2013;306:162–8. doi: 10.1016/j.tox.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Saito M, Kato Y, Ito E. et al. Expression screening of 17q12-21 amplicon reveals GRB7 as an ERBB2-dependent oncogene. FEBS Lett. 2012;586:1708–14. doi: 10.1016/j.febslet.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Wolff A, Hammond MEH, Hicks DG. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncoloty/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]