Scheme I.
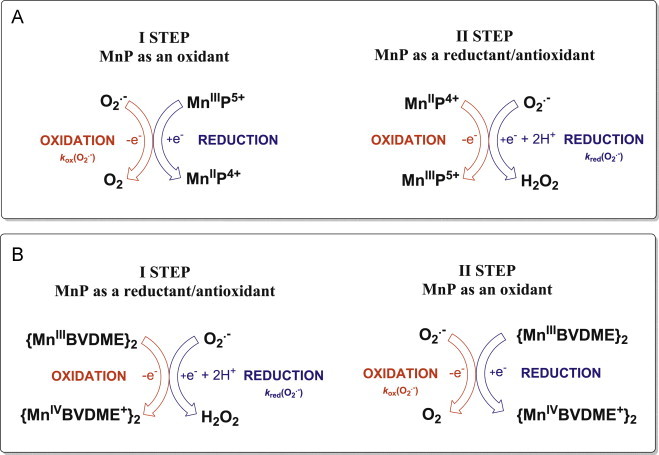
The O2•− dismutation process catalyzed by pentacationic Mn porphyrins (A) and Mn biliverdin (and its derivatives) (B). A. Dismutation of O2•− by pentacationic Mn complexes involves MnIII/MnII redox couple. The dismutation process shown here communicates important information that SOD mimics exhibit PRO- and ANTI-oxidative actions during O2•− dismutation, i.e. they can accept (1st step) electron from O2•− producing O2, and give away electron to another molecule of O2•− (2nd step) producing H2O2 if their metal-centered reduction potential, E1/2 for MnIIIP/MnIIP redox couple is within the range of +100 to +500 mV vs NHE. Such E1/2 values indicate that SOD mimics are mild oxidants and reductants/antioxidants and that their reduction potentials are biologically compatible and allow them to couple with numerous other biological targets. Yet, there is another side of dismutation process. The 2nd step (while antioxidative in its nature with regards to O2•−) produces an oxidant, H2O2. The SOD enzymes and their mimics may in turn be only viewed as anti-oxidative defense when (under physiological conditions) are coupled with multiple H2O2 removing systems. It explains why in numerous in vivo reactions (oxidation of thiols, ascorbate, lipids, NADPH, NADH, see below) MnP acts as pro-oxidant, while still GENERATING therapeutic EFFECTS. Thus the additional distinction must be made here between the actions of redox-active compounds and therapeutic effects observed. Please note that MnPs with very negative E1/2<0 mV vs NHE (very electron rich such as MnTBAP3− or MnTSPP3−) are fairly redox-inert and cannot be reduced in a 1st step with O2•−, but can act as anti-oxidants (reductants) during oxidation with strong oxidants (such as ONOO−, ClO−, lipid reactive species). Since they are electron-rich, and oxidation with those species involves their binding to Mn site, such reactions are less favored than those with electron-deficient Mn porphyrins (such as MnTE-2-PyP5+) with E1/2 in between +100 and +500 mV vs NHE [7]. Note, also that MnPs with E1/2>+450 (very electron-deficient, MnIIBr8TM-3(or 4)-PyP4+ and Cl5MnIITE-2-PyP4+), are stabilized in +2 oxidation state and lose Mn readily at pH 7.8. For comparison those Mn(III) N-alkylpyridylporphyrins with Mn in +3 oxidation state (indicated with III) resist even 98% sulfuric and 36% hydrochloric acids. Such compounds therefore start dismutation by reducing O2•− while oxidizing Mn in a 1st step, and get reduced to Mn(II) while oxidizing O2•− in a 2nd step, reversal of Scheme A. B. Dismutation of O2•− by Mn(III) biliverdin and its analogs employ MnIV/MnIII redox couple, shown in Scheme for Mn(III) biliverdin dimethylester (BVDME). Mn(III)- and Fe(III) corroles employ metal +3/+4 redox couple also [8]. In turn, in a 1st step those metal sites reduce O2•− while undergoing oxidation to M(IV) complex, whereas in backward reaction they get reduced with O2•− (and restored as catalysts) (see [3] for details). Regardless of which metal redox couple is used (+3/+2 or +3/+4), O2•− only cares if the metal easily accepts electron from and donates the electron to it, thus operating at E1/2 value that is around the midway potential between the oxidation and reduction of O2•−; consequently both steps of dismutation are facilitated to a similar extent. That indeed is true, the E1/2 ranges between +440 and +470 mV vs NHE for Mn(III) biliverdin analogs, while E1/2=+468 and +480 mV vs NHE for Br8MnIITM-3-PyP4+ for Br8MnIITM-4-PyP4+[9].
