Scheme II.
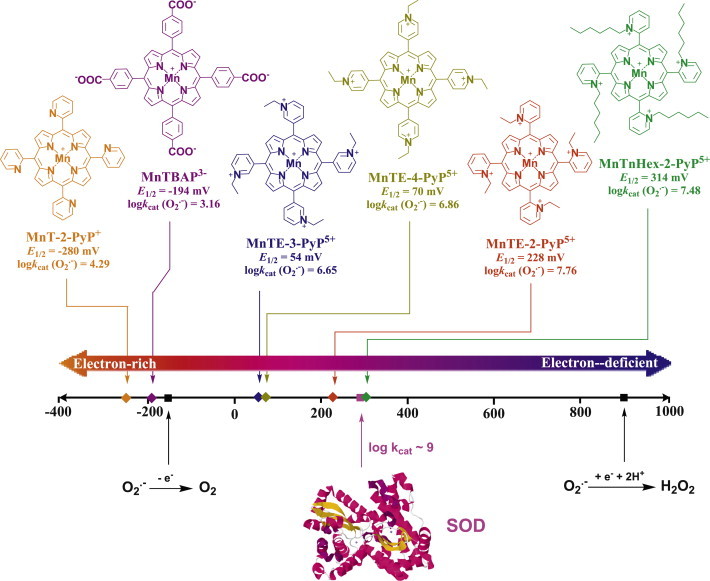
SOD mimics were designed in a process where the kinetics and thermodynamics of O2•− dismutation catalyzed by superoxide dismutases were mimicked. The kinetics of the catalysis O2•− dismutation relates to the electrostatic facilitation of the approach of anionic O2•− towards the metal site. In the case of enzyme the anionic superoxide is pulled towards the metal site through the tunnel of amino acids with cationic residues. Those MnPs which have cationic charges on periphery are ~2 or 2.5 orders of magnitude faster catalysts than those which are either neutral or anionic at the periphery, respectively. The thermodynamics relates to the redox properties of the metal site (described with metal-centered reduction potential, E1/2) which would allow Mn to equally easily accept electron from one O2•− and give it to another O2•−. Cationic compounds with E1/2 for MnIIIP/MnIIP redox couple around the E1/2 of SOD enzymes (~+300 mV vs NHE) were synthesized and demonstrated to efficiently catalyze O2•− dismutation in aqueous solution with high rate constants. In addition, they are extremely stable complexes towards the loss of Mn. Such E1/2 of SOD enzyme and its mimics sits around the midway potential between the E1/2 for O2•− reduction at +890 mV vs NHE and its oxidation at −180 mV vs NHE. In turn, it allows equal facilitation for both steps of dismutation process [3,9]. Indeed both steps occur with the same rate constants with SOD enzyme as was demonstrated for its mimic, MnTE-2-PyP5+[10]. The most powerful SOD mimics belong to the class of Mn(III) ortho (N-substituted pyridinium-2-yl)porphyrins. They possess both favorable electrostatics, as they bear 4 positive charges at the periphery to attract O2•−, and favorable thermodynamics, E1/2 ~+200 to +450 mV vs NHE. Among them the ortho, meta and para pyridyl MnPs which are β-halogenated (with chlorines at β positions of pyrrole rings, see Scheme IV) possess the highest kcat(O2•−), close to that of SOD enzymes; yet they are the least stable. Adapted from [3].
