Scheme IX.
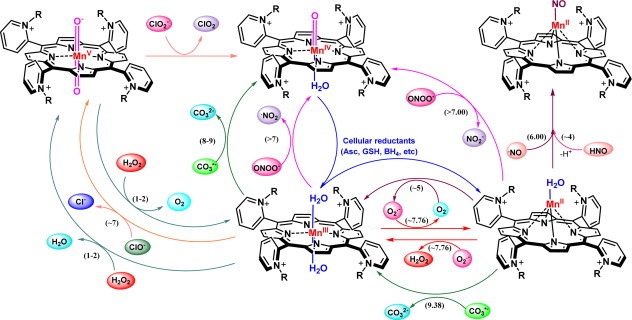
Reactivity of Mn(III) ortho N-substituted pyridylporphyrins towards different low-molecular weight reactive species. In late 1990s, along with growing awareness of highly oxidizing species, ONOO–, it became obvious that all potent SOD mimics can effectively reduce ONOO− either one- (if starting from MnIIIP) or two-electronically (if starting from MnIIP), producing either highly oxidizing •NO2 or benign NO2−, respectively. The ONOO− reduction by MnP is only few-fold slower than the catalysis of O2•− dismutation, demonstrating that all potent SOD mimics are potent peroxynitrite scavengers. Compounds which cannot dismute O2•−, such as MnTBAP3−, could be oxidized with strong oxidant, such as ONOO−, in a 1st step and reduced with either ascorbate or thiol or O2•− in a 2nd step. If O2•− is involved in a 2nd step, such compounds can affect in vivo levels of O2•−. Yet, the lower the SOD-like activity, the lower is the ability to reduce ONOO−. Indeed, MnTBAP3− is ~2 orders of magnitude less able reductant of ONOO− than is MnTE-2-PyP5+. Coupling of ONOO− reduction to O2•− oxidation, has been explored with MnTM-4-PyP5+[25], and may be operative with other compounds. Coupling of ONOO− reduction to cellular reductants is likely operative with potent SOD mimics also (Scheme X). Due to high levels of carbonate in vivo, ONOO− forms an adduct with CO2. The ONOOCO2− adduct degrades to CO3•− and •NO2 radicals. Due to its radical nature, all potent SOD mimics react rapidly with CO3•− in a pH-dependent manner, log kred(CO3•-) ranging between 8 and 9 (Scheme X, Eqs. 5 and 6). Most of the rate constants provided here were based on those determined for MnTE-2-PyP5+. The linear relationship between log kcat(O2•−) and log kred(ONOO−) allows for calculations of other kred(ONOO−) (see Scheme XIV).
