Scheme XI.
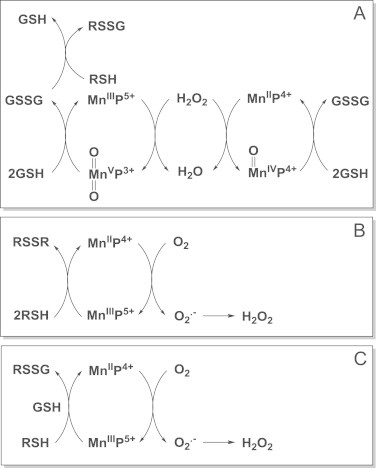
Interaction of MnPs with thiols/H2O2 in thiol oxidase or GPx fashion. While catalase-like action may be of no in vivo relevance due to low kcat(H2O2), the key role of H2O2 in the actions of MnPs, other than catalase-like, has already been established and occur in reactions of MnPs with cellular reductants (see also Schemes XIII and XIX). Note that H2O2 is reduced to water (H2O2 is eliminated) in Scheme A, while it is generated in Schemes B and C. The GSSG species can exchange thiol spontaneously with RSH whereby glutathionylated protein is formed. Reactivity towards thiols may predominate in therapeutic effects of MnPs. Studies on CNS injuries, diabetes and cancer show that oxidation of cysteines of p50 and p65 subunits by MnP (enhanced with dexamethasone as a producer of H2O2) suppresses/precludes the NF-κB activation and in turn suppresses inflammation rescuing the diseased cell and/or precluding the survival of cancer cell [45–49]. In addition S-glutathionylation of cysteines of complexes I, III and IV and subsequent inactivation of complexes I and III of mitochondrial respiration may play key role in reducing ATP levels in tumor cell whereby suppressing tumor growth (see Scheme XX) [46]. The impact of MnP on ATP levels via glycolysis has been demonstrated also [50] (Scheme XVII) MnIIIP5+ can either oxidize thiols directly (B and C), undergoing one-electron reduction to MnIIP4+ whereby thiyl radical is formed in a thiol oxidase fashion; or it can act in GPx fashion while coupling with H2O2 to S-glutathionylate thiols (A and C). Indeed, studies on lymphoma cells showed clearly that S-glutathionylation happens only in the presence of H2O2 and GSH [45,47]. The H2O2 may originate either from: (i) already increased oxidative stress of a cancer cell, or (ii) additional generation via radio- or chemotherapy, or (iii) catalytic cycling of MnP with ascorbate or thiols.
