Scheme XX.
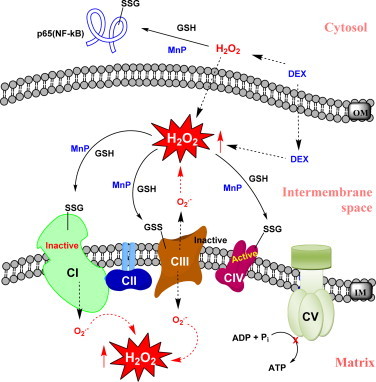
MnP inactivates complexes I and III via their S-glutathionylation when combined with dexamethasone in lymphoma cellular model. MnTE-2-PyP5+ was tested in lymphoma cellular model where it enhanced cell killing in the presence of dexamethasone via enhancing H2O2 production [45,46]. H2O2 in turn was employed by MnP to catalyze S-glutathionylation of protein thiols. Such action, depicted in Scheme XI, is in essence either GPx- or thiol oxidase-like activity of MnP. Such oxidative modification has been demonstrated on both cytosolic and mitochondrial levels. In cytosol cysteine units of p50 and most so p65 of NF-kB seem to be modified, while complexes I, III and IV were S-glutathionylated in mitochondria. Also oxidation of p50 by MnP in nucleus has been suggested by Piganelli's group [48]. All proteins except complex IV (though S-glutathionylated) were inactivated by such oxidative modifications. That indicates that MnP under conditions of high oxidative stress, i.e. high H2O2 levels, which may be produced by different treatment modalities such as radiation and chemotherapy (dexamethasone or ascorbate or temozolomide, [3,41,42,44,45,47,53], would suppress anti-apoptotic, survival pathways and cellular energetics, killing cancer cell on two metabolic fronts. Adapted from [45,46].
