Scheme XXIII.
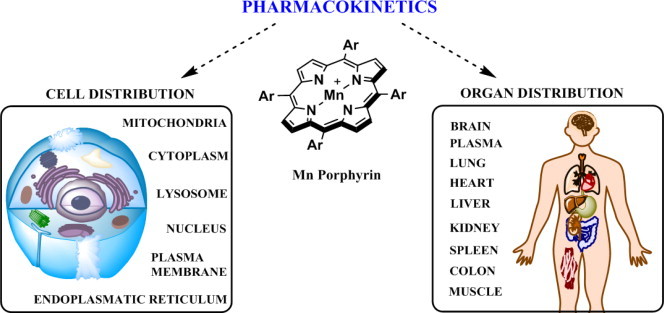
Pharmacokinetic and bioavailability studies with MnPs. Bioavailibilities of MnPs depend upon their lipophilicities, polarities, charge, size, shape, and bulkiness. Comprehensive studies have been reported on MnPs that bear similar redox properties (MnTE-2-PyP5+, MnTM-2-PyP5+, MnTnBu-2-PyP5+, MnTnHex-2-PyP5+ and MnTnBuOE-2-PyP5+ in different organisms via various administration routes. The PK studies involved plasma and different organs such as liver, kidney, lungs, spinal cord, brain, spleen, heart, colon, salivary glands and tongue. Also intracellular accumulation has been addressed on ZnPs and MnPs and data published on membrane, cytosolic, nuclear, mitochondrial, endoplasmatic reticular and lysosomal accumulation [78–83]. Largest body exposure of MnPs, expressed as AUC (area under pharmacokinetic curve) has been obtained via subcutaneous route; this route also has another advantage as it imposes the lowest acute side effects in MnP administration − blood pressure drop. Highest levels of MnPs are found in liver and kidneys (at µM levels), while the lowest were detected in brain (at nM levels). Lipophilic and cationic, MnTnHex-2-PyP5+ and MnTnBuOE-2-PyP5+ accumulate several fold more in brain (driven there by the anionic phospholipids) than does hydrophilic MnTE-2-PyP5+ [Spasojevic, Tovmasyan, Weitner et al., unpublished]. Given the highly positive charge, MnTE-2-PyP5+ accumulates ~3-fold more in nucleus than in cytosol of macrophages and LPS stimulated macrophages [48]. Such accumulation seems to be important given the suggestion by Tse and Piganelli that MnP oxidizes p50 in nucleus [48,49]. Cationic MnPs were also found to favor accumulation in cell wall of E. coli due to abundance of negatively charged phospholipids [84]. Extensive distribution studies of fluorescent Zn analogs using confocal fluorescent microscopy indicate their accumulation in membranes, lysosomes and endoplasmatic reticulum also [78,85]. The study was carried out on adenocarcinoma cells where lipophilic ZnTnHex-2-PyP5+ was also found in mitochondria in the vicinity of cytochrome c oxidase [79]. The only difference between Zn and Mn analogs is the lack of single charge on Zn center. Yet, with longer alkyl analogs, such as MnTnHex-2-PyP5+ no difference in their lipophilicities exists as single cationic charge on Mn is shielded from the solvent by long hexyl chains. Moreover, in vivo MnPs get readily reduced with cellular reductants, lose single charge on metal site and become similarly hydrophilic as ZnPs [86]. Thus, accumulation data on ZnPs (in particular those with long alkylpyridyl substituents) can be readily applied to MnPs.
