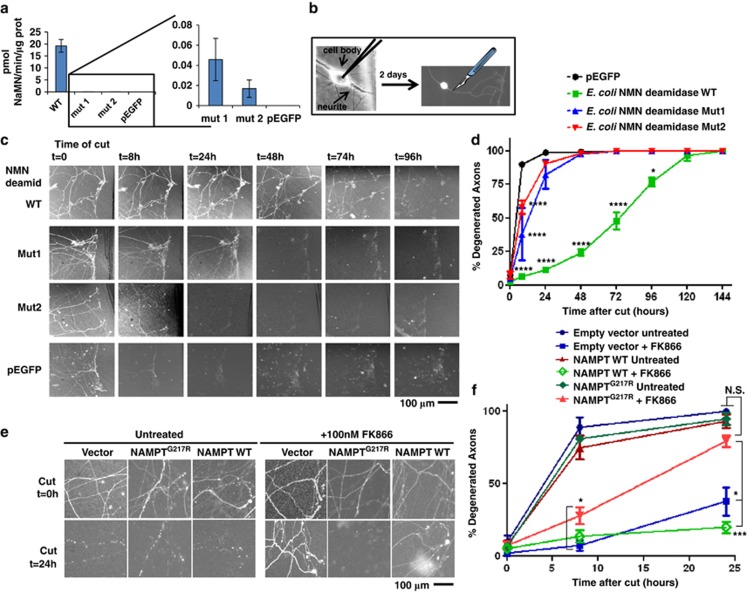
Figure 3.
Genetic evidence supporting the role of NMN and NAMPT in axon degeneration. (a) Plasmid cDNA constructs encoding E. coli NMN deamidase WT or its virtually enzymatic inactive mutants Mut1 and Mut2 fused to EGFP, or pEGFP vector alone, were transfected in HEK293T cells. Cells were harvested 48 h after transfection, lysed and NMN deamidase activity was measured as described in Materials and Methods. Activity of the enzymatically inactive mutants was barely detectable (left panel), but on expanding the y axis it was clearly detectable with respect to cell lysates transfected with pEGFP vector alone (right panel) (n=3, mean and S.D. shown). (b) Schematic representation of microinjection experiment. Nuclei of dissociated SCGs were microinjected with the plasmid together with pDsRed2-N1 for expression of DsRed2 fluorescent marker to visualize individual transfected neurons. Two days after microinjections, fluorescent neurites were cut and imaged at different time points. (c and d) NMN deamidase strongly delays degeneration of cut SCG neurites. (c) Constructs expressing WT NMN deamidase or mutants with disrupted enzymatic activity fused to EGFP were microinjected into SCG nuclei along with pDsRed2-N1. EGFP fluorescence within the neurites confirmed expression and homogeneous localization of the expressed proteins in cell bodies and axons. SCG neurites were cut 48 h after microinjection, and DsRed2 or EGFP images were acquired at different time points after cut as indicated. (d) The percentage of fluorescent-degenerated axons was calculated in five fields of three independent experiments. (mean ±S.E.M., n=15, one-way ANOVA followed by Bonferroni's post-hoc test *P<0.05, ****P<0.0001). (e and f) Drug-resistant NAMPT restores rapid axon degeneration. (e) Representative images of SCG neurites 0 and 24 h after cutting and microinjected as in panel (b) with empty vector or with vectors expressing drug-resistant or WT NAMPT. SCGs were left untreated or 100 nM FK866 was added just after transection as indicated. (f) The percentage of fluorescent degenerated axons was calculated in 3–6 fields of three independent experiments as in panel (e). The results show that drug-resistant NAMPTG217R expression restores rapid axon degeneration after FK866 treatment partially at 8 h and totally at 24 h after cut (mean±S.E.M., n=6–18, one-way ANOVA followed by Bonferroni's post-hoc test *P<0.05, ***P<0.001). NS, not significant