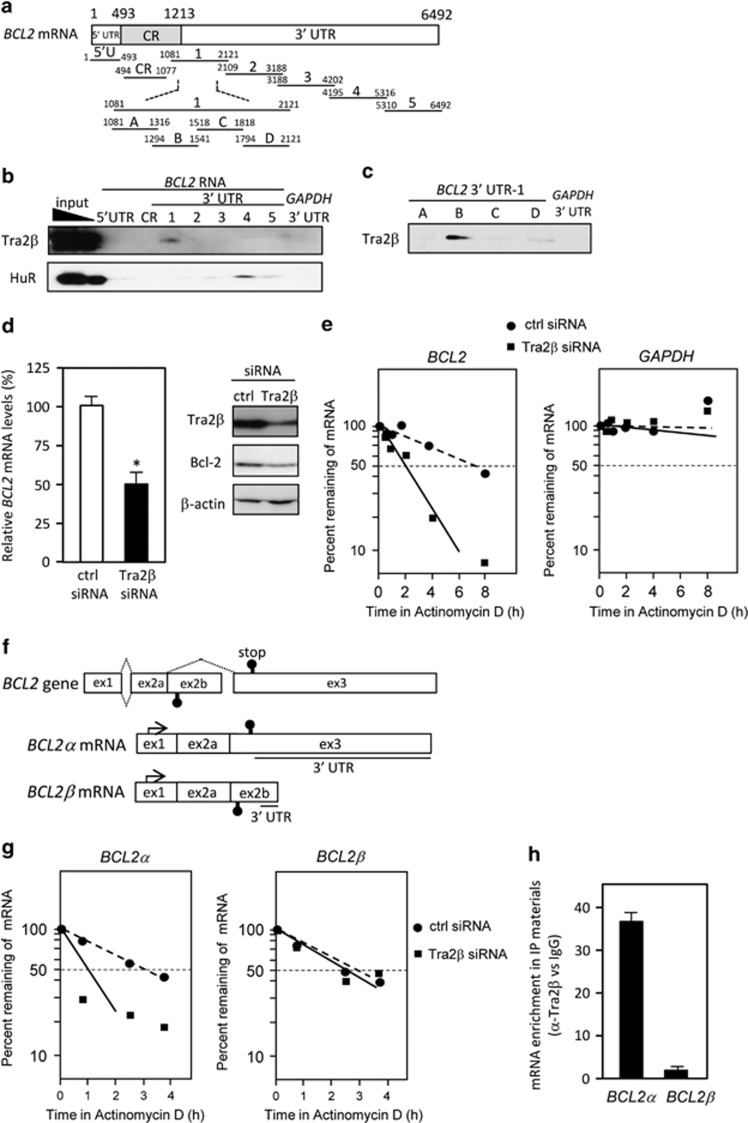
Figure 2.
Tra2β binds to BCL2 3′ UTR. (a) Schema of the 5′ UTR, coding region (CR), and 3′ UTR fragments of BCL2 mRNA that were used for in vitro binding assays. (b) A biotin pull-down assay was carried out using lysates prepared from HCT116 cells and biotinylated RNA fragments. RNA–protein complexes were isolated with paramagnetic streptavidin-conjugated Dynabeads (Dynal), and bound Tra2β or HuR was detected by western blotting. (c) After biotin pull-down using biotinylated fragments of the 3′ UTR of BCL2, bound Tra2β was analyzed by western blotting. (d, left) Forty-eight hours after transfection with 10 nM control (ctrl) or TRA2β siRNA, the amounts of BCL2 and GAPDH mRNA were determined by qPCR. The values shown are the mean±S.D. (n=5). *Significantly decreased compared with control siRNA-treated cells (P<0.05 by unpaired Student's t-test). (d, right) After treatment of HCT116 cells with 10 nM control or TRA2β siRNA for 48 h, levels of Tra2β and Bcl-2 were measured by western blotting. β-actin was used as a loading control. (e) After HCT116 cells were transfected with control or TRA2β siRNA for 48 h, they were treated with actinomycin D (2.5 μg/ml) for the indicated times. BCL2 and GAPDH mRNA levels were measured by qPCR. (f) Schema of two BCL2 mRNA isoforms, BCL2α and BCL2β. The arrow indicates the transcriptional start site. The 3′ UTRs of these isoforms are underlined. (g) After a 48-h transfection of control or TRA2β siRNA, HCT116 cells were treated with actinomycin D (2.5 μg/ml) for the indicated times. BCL2α and BCL2β mRNA levels were measured by qPCR and the percentage of mRNA that remained was plotted. (h) qPCR was used to measure the abundance of BCL2α or BCL2β mRNAs present in the Tra2β-IP materials after the RIP assay was measured by qPCR