FIGURE 6.
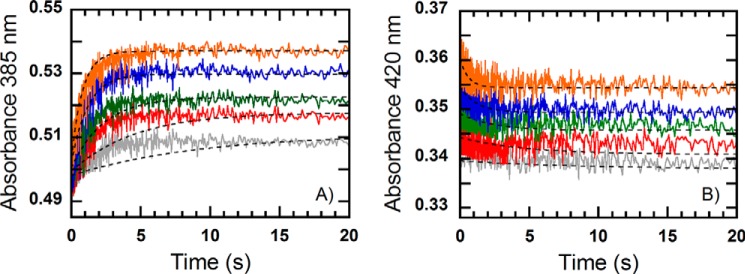
Kinetics of the reaction of a pre-mixed solution of MCRred1 (18 μm) and CoB7SH (18 μm) with methyl-SCoM at concentrations of 0.125, 0.25, 0.5, 1, and 2 mm (final concentrations) in 50 mm Tris-HCl pH 7.6. The reactions were performed under anaerobic conditions using the stopped-flow spectrophotometer at 25 °C and monitored at 385 (A) and 420 nm (B). The traces were fit to a single exponential equation. The dashed lines represent simulations based on the model presented in Scheme 2 with kinetic parameters listed in Table 1 and the following extinction coefficients: ϵ385(MCR(NiI)) = 37,340 m−1 cm−1; ϵ385(MCR(NiI)·CH3SCoM) = 35,040 m−1 cm−1; ϵ385(CoB7SH·MCR(NiI)·CH3SCoM) = 30,010 m−1 cm−1; ϵ385(MCR(NiII)·CoB7S−·SCoM) = 25,490 m−1 cm−1; ϵ385(MCR(NiI)·CoB7SH) = 35,790 m−1 cm−1; ϵ385(CoB7SH·MCR(NiI)·CH3SCoM*) = 35,550 m−1 cm−1; ϵ420(MCR(NiI)) = 18,060 m−1 cm−1; ϵ420(MCR(NiI)·CH3SCoM) = 20,620 m−1 cm−1; ϵ420(CoB7SH·MCR(NiI)·CH3SCoM) = 20,550 m−1 cm−1; and ϵ420(MCR(NiII)·CoB7S−·SCoM) = 20,100 m−1 cm−1; ϵ420(MCR(NiI)·CoB7SH) = 21,070 m−1 cm−1; and ϵ420(CoB7SH·MCR(NiI)·CH3SCoM*) = 22,920 m−1 cm−1.
