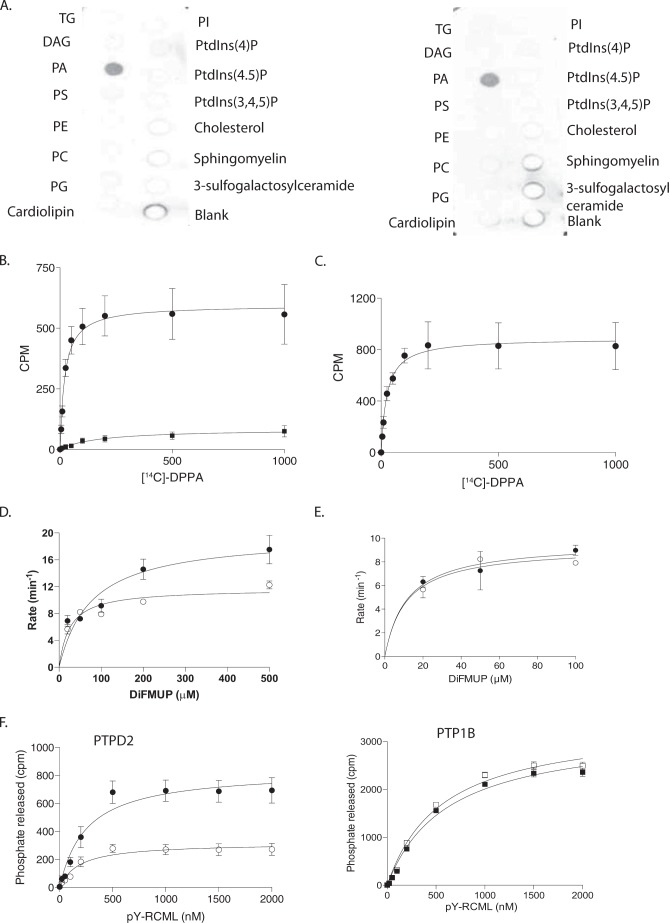
FIGURE 8.
Phosphatidic acid bound PTPD2 in vitro and augmented its catalytic activity. A, protein-lipid overlay to measure binding of the catalytic domain of PTPD2 to a lipid array. Strips containing the indicated phospholipids were probed with 0.5 μg/ml of bacterially expressed, His-tagged catalytic domain of wild type (left) or substrate-trapping mutant form (right) of PTPD2. Bound protein was detected by immunoblotting with anti-His antibody. B, recombinant PTPD2 catalytic domain (●) or PTP1B (■) was incubated with various concentrations of [14C]DPPA, and the PTP-[14C]DPPA complex was separated from unbound lipid by centrifugation, and bound radioactivity was measured by scintillation counting. C, [14C]DPPA binding to full-length PTPD2 performed as in B. D, recombinant PTPD2 catalytic domain was left untreated (○) or incubated with phosphatidic acid for 30 min (●), and the phosphatase activity was measured using DiFMUP as substrate. E, effect of phosphatidylserine on PTPD2 catalytic activity was assessed as in D. F, PTPD2 (left) or PTP1B (right) were left untreated (open symbols) or incubated with [14C]DPPA (closed symbols) for 30 min, and the phosphatase activity was measured using 32P-reduced carboxamidomethylated and maleylated lysozyme (RCML) as substrate.