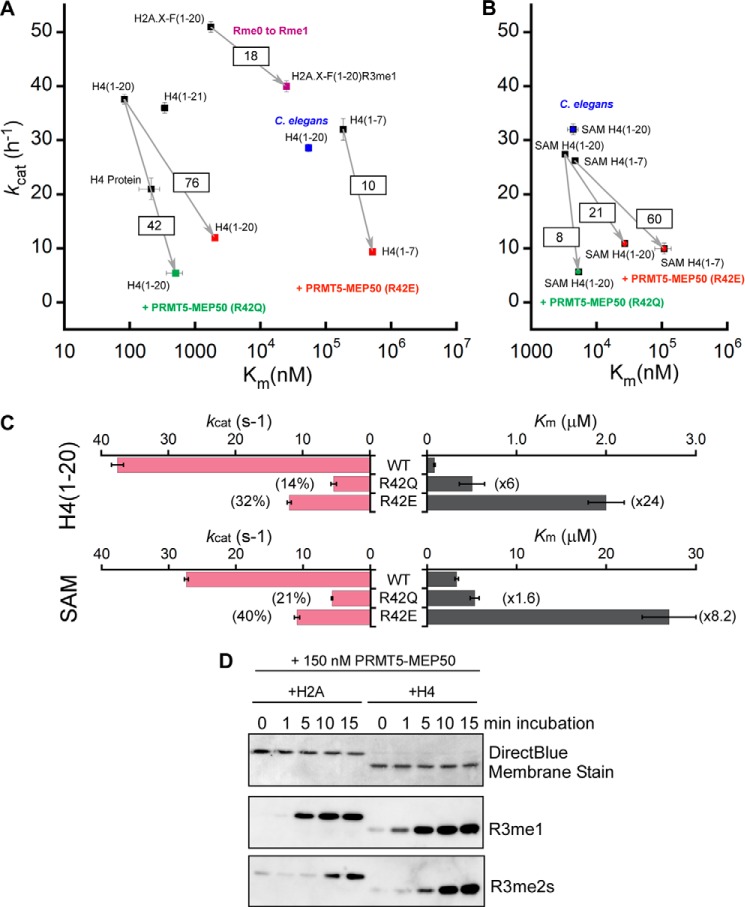
FIGURE 6.
Substrate specificities for XlPRMT5-MEP50 and the impact on enzymatic efficiency upon mutation of MEP50 insertion loop residue Arg-42. Kinetic parameters for the various tested substrates (histone H4, histone peptides, and SAM) are plotted, with the kcat (h−1) on the y axis and the Km (nm; logarithmic scale) on the x axis. Highest enzymatic efficiencies are obtained with substrates found in the top left quadrant, whereas low enzymatic efficiencies are obtained with substrates found in the opposite bottom right quadrant. Arrows indicate the loss (squared values) of enzymatic efficiency upon arginine monomethylation (purple) or upon mutation of MEP50 residue Arg-42 to glutamic acid (red) and to glutamine (green). For reference, enzymatic behavior of CePRMT5 is represented in blue. A, representation of kinetic parameters for histone substrates using saturating concentration of SAM. B, representation of kinetic parameters for SAM substrate using saturating concentration of histone substrates. C, impact of XlMEP50R42Q and XlMEP50R42E on catalytic turnover (kcat; pink bars) and substrates' affinities (Km; gray bars) for both peptide and SAM substrates. The decrease of methyl transfer is represented as a percentage of wild-type kcat, whereas the loss of affinity is given as -fold increase of wild-type Km. D, histones H2A or H4 were incubated with XlPRMT5-MEP50 and SAM. Reactions were stopped at 0, 1, 5, 10, and 15 min with the addition of SDS-polyacrylamide gel loading buffer and heating to 100 °C. Reaction products were immunoblotted with monomethylarginine (R3me1)- or symmetric dimethylarginine (R3me2s)-specific antibodies.