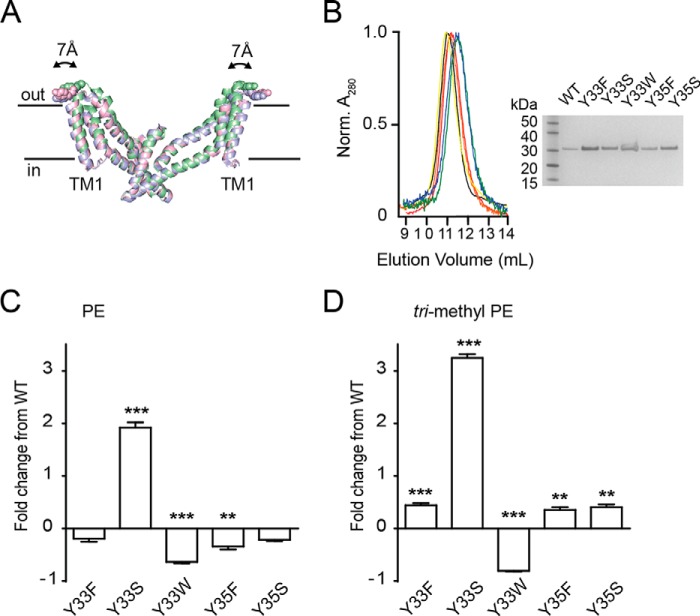
FIGURE 5.
Functional properties of tyrosine 33 mutants. A, superimposition of the trimerization domain of the outward facing (green; Protein Data Bank code 2NWX), intermediate outward facing (pink; Protein Data Bank code 3V8G), and inward facing (blue; Protein Data Bank code 3KBC) crystal structures. Structures were aligned to TM4. The transport domain was omitted for clarity. Tyr-33 is shown as spheres. The figure was made using the program PyMOL (38). B, size exclusion column profile for wild-type GltPh (black), Y33F (red), Y33S (orange), Y33W (yellow), Y35F (green), and Y35S (blue). Inset, SDS-PAGE of purified wild-type GltPh (lane 2), Y33F (lane 3), Y33S (lane 4), Y33W (lane 5), Y35F (lane 6), and Y35S (lane 7). Lane 1 contains ladder. C, -fold change in l-[3H]aspartate transport rates for wild-type and mutant GltPh reconstituted in PE liposomes. D, -fold change in l-[3H]aspartate transport rates for wild-type and mutant GltPh reconstituted in trimethyl PE liposomes. Data represent the mean of experiments performed in triplicate, and error bars indicate S.E. with p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***) compared with WT in each lipid species.