FIGURE 2.
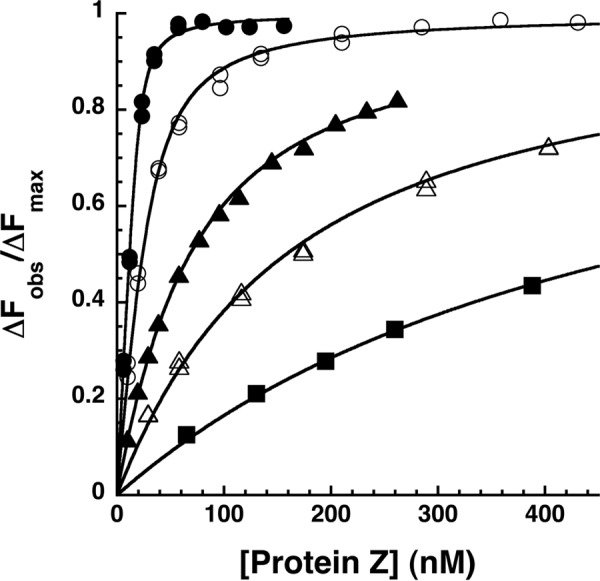
Equilibrium binding titrations of the NBD-ZPI-PZ interaction at varying ionic strengths. Fluorescence titrations of NBD-ZPI (25 nm) in pH 7. 1 Tris buffer containing 0.1 (●), 0.175 (○), 0.25 (▴), 0.35 (▵), and 0.5 (■) M NaCl at 25 °C were monitored at λex 480 nm and λem 545 nm as a function of increasing PZ concentration. Observed fluorescence values were corrected for buffer and dilution and then fit by the quadratic equilibrium binding equation (solid lines) with the binding stoichiometry fixed at values fitted from separate stoichiometric titrations as described under “Experimental Procedures.” The observed changes in fluorescence (ΔFobs) are expressed relative to the fitted maximal fluorescence change (ΔFmax), the latter showing no significant dependence on ionic strength within experimental error.
