FIGURE 3.
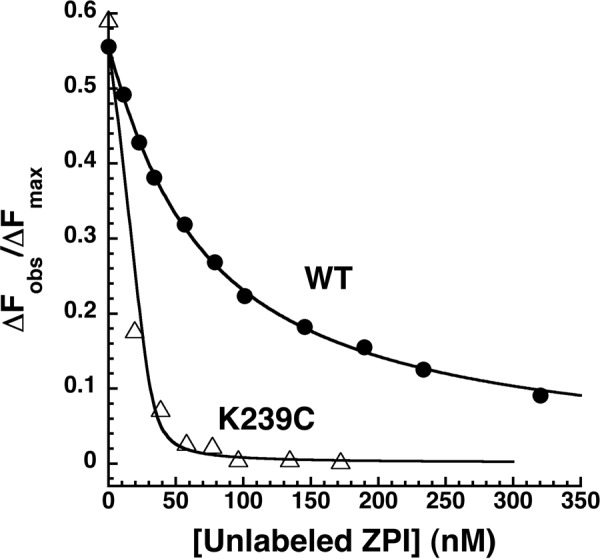
Competitive equilibrium binding titrations of the NBD-ZPI-PZ complex with wild-type and K239C ZPI. NBD-ZPI-PZ complex formed with 53 nm NBD-ZPI and 32 nm PZ was titrated with increasing concentrations of unlabeled wild-type and K239C ZPIs in pH 7. 1, I 0.15 Tris buffer at 25 °C, and the observed fluorescence was monitored at 545 nm as in Fig. 2. Fluorescence data corrected for buffer and dilution were fit by the cubic competitive binding equation with the binding stoichiometry and KD for the labeled ZPI interaction fixed at independently measured values and assuming a 1:1 binding stoichiometry for the unlabeled ZPIs as described under “Experimental Procedures.” Observed changes in fluorescence are expressed relative to the fitted maximal fluorescence change of the NBD-ZPI-PZ interaction (ΔFmax).
