FIGURE 6.
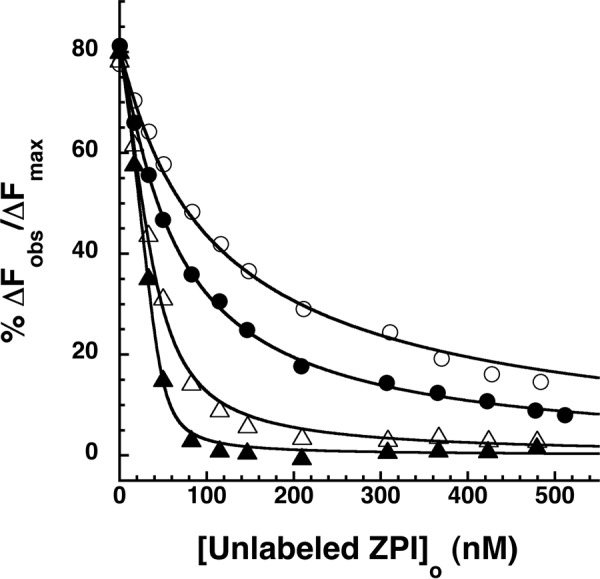
Effect of wild-type and K239A ZPI complexation with factor Xa on ZPI-PZ affinity. Competitive equilibrium binding titrations of NBD-ZPI-PZ complex formed with 55 nm NBD-ZPI and 49 nm PZ with wild-type ZPI (circles) or K239A ZPI (triangles) before (closed symbols) and after reaction with 1 SI eq of factor Xa (open symbols) are shown. Titrations were performed in pH 7.1, I 0.15 Tris buffer at 25 °C, and the observed fluorescence changes were corrected for buffer and dilution. Data were fit by the cubic competitive binding equation as in Fig. 3 (solid lines), and ΔFobs was expressed relative to the fitted maximal fluorescence change.
