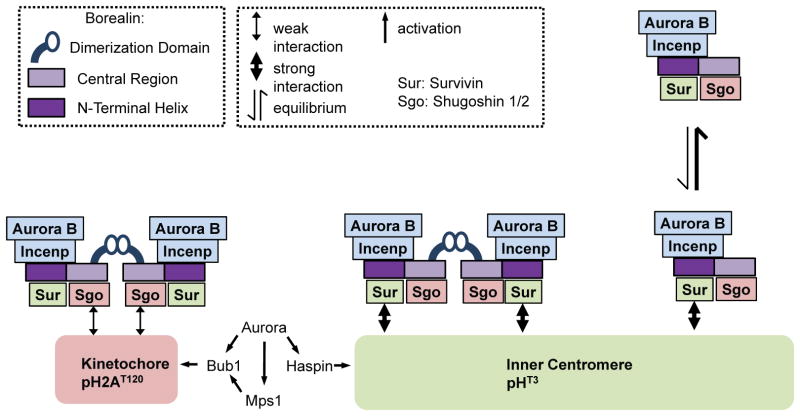
Figure 10. Model for the role of dimerization in CPC interactions with the centromere.
Dimeric CPC stably binds at inner centromeres possibly via two Survivin-pH3T3 interactions mediated by Borealin dimerization. Removing the dimerization domain of Borealin results in rapid exchange at inner centromeres since monomeric CPC might only contact a single pH3T3 molecule. When endogenous Borealin is knocked-down, Borealin mutants lacking dimerization transiently associate with the centromere to provide partial function. However, when endogenous Borealin is present, dimerization-defective mutants compete for other CPC family members to increase their exchange and act as dominant negatives. At the kinetochore, dimeric CPC binds with a high rate of turnover potentially via dual pH2AT120-Sgo1/2 interactions. These kinetochore-interactions are too weak to allow association in the context of a monomeric CPC. According to the prevailing model of CPC targeting, CPC interacts simultaneously with pH3T3 via Survivin and pH2AT120 via Sgo1/2. Our results do not preclude simultaneous binding, but do suggest that pH3T3 would contribute most of the binding energy if this binding mode occurs. Theoretically, CPC monomers or dimers could simultaneously interact with single pH3T3 and pH2AT120.