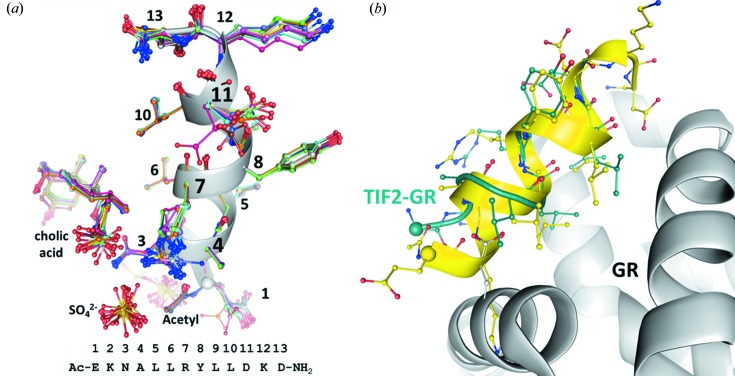
Figure 7.
TIF2 conformations in the superhelix and when bound to GR. (a) Superposition of all 13 protomers. The residues are numbered according to the sequence given at the bottom. The main-chain torsion angles of residues 12 and 13 deviate from α-helical geometry. Because of weak electron density, these residues were modelled with half occupancy. Clashes of these residues with the same residues of neighbouring helices indicate that the crystal actually contained a mixture of peptides. (b) The GR/dexamethasone/TIF2 complex (Seitz et al., 2010 ▶) is superimposed with one representative protomer (coloured yellow) from the superhelix. GR is coloured grey and the TIF2 peptide in complex with GR is shown in dark green. The N-termini of the peptides are marked by spheres. The N-terminal part of TIF2 cannot adopt an α-helical conformation when binding to GR due to clashes with the receptor.